Page 524 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 524
Polypyrrole-based nanocomposite adsorbents 477
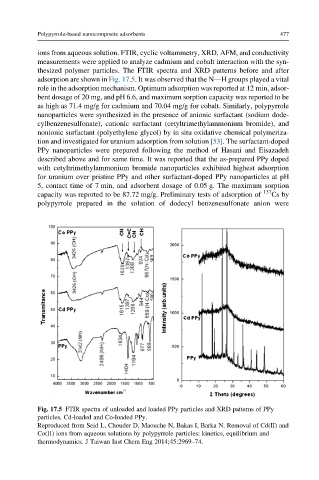
ions from aqueous solution. FTIR, cyclic voltammetry, XRD, AFM, and conductivity
measurements were applied to analyze cadmium and cobalt interaction with the syn-
thesized polymer particles. The FTIR spectra and XRD patterns before and after
adsorption are shown in Fig. 17.5. It was observed that the NdH groups played a vital
role in the adsorption mechanism. Optimum adsorption was reported at 12 min, adsor-
bent dosage of 20 mg, and pH 6.6, and maximum sorption capacity was reported to be
as high as 71.4 mg/g for cadmium and 70.04 mg/g for cobalt. Similarly, polypyrrole
nanoparticles were synthesized in the presence of anionic surfactant (sodium dode-
cylbenzenesulfonate), cationic surfactant (cetyltrimethylammonium bromide), and
nonionic surfactant (polyethylene glycol) by in situ oxidative chemical polymeriza-
tion and investigated for uranium adsorption from solution [53]. The surfactant-doped
PPy nanoparticles were prepared following the method of Hasani and Eisazadeh
described above and for same time. It was reported that the as-prepared PPy doped
with cetyltrimethylammonium bromide nanoparticles exhibited highest adsorption
for uranium over pristine PPy and other surfactant-doped PPy nanoparticles at pH
5, contact time of 7 min, and adsorbent dosage of 0.05 g. The maximum sorption
capacity was reported to be 87.72 mg/g. Preliminary tests of adsorption of 137 Cs by
polypyrrole prepared in the solution of dodecyl benzenesulfonate anion were
Fig. 17.5 FTIR spectra of unloaded and loaded PPy particles and XRD patterns of PPy
particles, Cd-loaded and Co-loaded PPy.
Reproduced from Seid L, Chouder D, Maouche N, Bakas I, Barka N. Removal of Cd(II) and
Co(II) ions from aqueous solutions by polypyrrole particles: kinetics, equilibrium and
thermodynamics. J Taiwan Inst Chem Eng 2014;45:2969–74.