Page 529 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 529
482 Polymer-based Nanocomposites for Energy and Environmental Applications
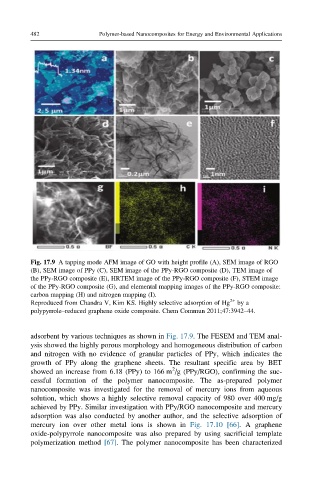
Fig. 17.9 A tapping mode AFM image of GO with height profile (A), SEM image of RGO
(B), SEM image of PPy (C), SEM image of the PPy-RGO composite (D), TEM image of
the PPy-RGO composite (E), HRTEM image of the PPy-RGO composite (F), STEM image
of the PPy-RGO composite (G), and elemental mapping images of the PPy-RGO composite:
carbon mapping (H) and nitrogen mapping (I).
Reproduced from Chandra V, Kim KS. Highly selective adsorption of Hg 2+ by a
polypyrrole–reduced graphene oxide composite. Chem Commun 2011;47:3942–44.
adsorbent by various techniques as shown in Fig. 17.9. The FESEM and TEM anal-
ysis showed the highly porous morphology and homogeneous distribution of carbon
and nitrogen with no evidence of granular particles of PPy, which indicates the
growth of PPy along the graphene sheets. The resultant specific area by BET
2
showed an increase from 6.18 (PPy) to 166 m /g (PPy/RGO), confirming the suc-
cessful formation of the polymer nanocomposite. The as-prepared polymer
nanocomposite was investigated for the removal of mercury ions from aqueous
solution, which shows a highly selective removal capacity of 980 over 400 mg/g
achieved by PPy. Similar investigation with PPy/RGO nanocomposite and mercury
adsorption was also conducted by another author, and the selective adsorption of
mercury ion over other metal ions is shown in Fig. 17.10 [66]. A graphene
oxide-polypyrrole nanocomposite was also prepared by using sacrificial template
polymerization method [67]. The polymer nanocomposite has been characterized