Page 531 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 531
484 Polymer-based Nanocomposites for Energy and Environmental Applications
heating at 90°C. The pyrrole-impregnated carbon was then added to the oxidant solu-
tion to complete polymerization. The as-prepared polymer nanocomposite was inves-
tigated for heavy metal adsorption and found to exhibit improved complexation
affinity for heavy metal ions due to the amine groups of polypyrrole.
17.14 Adsorption by copolymer nanofiber adsorbents
Sometimes, two different monomers can be polymerized to form polymer composite
(copolymer) that has also been proved efficient for adsorption studies. Such effort was
carried out by Javadian [71] when he synthesized polyaniline/polypyrrole copolymer
nanofibers for the removal of cobalt from aqueous solution. The adsorbent was pre-
pared by in situ chemical polymerization method in the presence of H 2 SO 4 as a dopant
and characterized by FESEM, TEM, FTIR, TGA, DSC, and BET surface area. The
as-prepared adsorbent demonstrated to be potentially viable for cobalt ion adsorption
with 99.68% at the optimum conditions of pH 7, 11 min contact time, 0.11 g adsorbent
dosage, and 100 mg/L initial concentration.
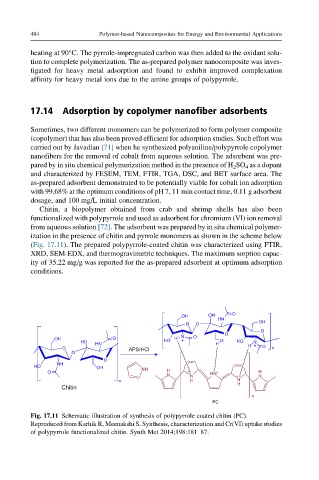
Chitin, a biopolymer obtained from crab and shrimp shells has also been
functionalized with polypyrrole and used as adsorbent for chromium (VI) ion removal
from aqueous solution [72]. The adsorbent was prepared by in situ chemical polymer-
ization in the presence of chitin and pyrrole monomers as shown in the scheme below
(Fig. 17.11). The prepared polypyrrole-coated chitin was characterized using FTIR,
XRD, SEM-EDX, and thermogravimetric techniques. The maximum sorption capac-
ity of 35.22 mg/g was reported for the as-prepared adsorbent at optimum adsorption
conditions.
OH OH O
HN
O O OH
O
O
OH O HO H N + O O
HO HN H • HO N
O APS/HCl H + O n
O
O
NH
HO OH NH
O H + HN + H
N N + N
n H N
H
Chitin
n
PC
Fig. 17.11 Schematic illustration of synthesis of polypyrrole coated chitin (PC).
Reproduced from Karhik R, Meenakshi S. Synthesis, characterization and Cr(VI) uptake studies
of polypyrrole functionalized chitin. Synth Met 2014;198:181–87.