Page 628 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 628
580 Polymer-based Nanocomposites for Energy and Environmental Applications
and coworkers [48] applied an electrochemical method for extraction of copper from
drinking water using polypyrrole/humic acid composite electrode.
21.6 Treatment of water
21.6.1 Removal of heavy metals
The term heavy metal refers to any metallic element in the periodic table that has a
3
relatively high density (>5.0 g/cm ), high atomic weight (>65), and is toxic or poi-
sonous at low concentrations (traces amounts, e.g., in ppm or ppb). As trace elements,
some heavy metals (e.g., Cu, Se, and Zn) are essential to maintain the metabolism of
the human body. However, at higher concentrations, they can lead to poisoning.
Heavy metal poisoning could result, for instance, from drinking water contamination,
high ambient air concentrations, or intake via the food chain. Some of the toxic heavy
metal ions are Hg(II), Cd(II), Pb(II), Cr(VI), Cu(II), As (III), etc.
Metal ions from wastewater can either be removed by filtration through polymer
nanocomposite membranes or by adsorption technique. However, adsorption technique
is the most efficient and economical. For water purification, nanocomposites are nor-
mally dispersed into water/wastewater for adsorption. The nanocomposites are then
separated by filtration or centrifugation. If the nanocomposites are magnetic, they
can be separated with the help of a magnet. Polypyrrole (PPy)-based nanocomposites
have been extensively used [49].Fe 3 O 4 -coated polypyrrole (PPy) magnetic
nanocomposite (MNC) is reported to be an effective adsorbent for the removal of
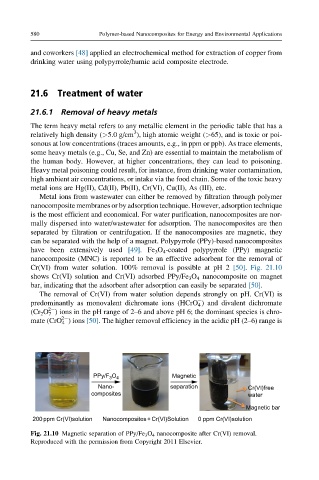
Cr(VI) from water solution. 100% removal is possible at pH 2 [50]. Fig. 21.10
shows Cr(VI) solution and Cr(VI) adsorbed PPy/Fe 3 O 4 nanocomposite on magnet
bar, indicating that the adsorbent after adsorption can easily be separated [50].
The removal of Cr(VI) from water solution depends strongly on pH. Cr(VI) is
predominantly as monovalent dichromate ions (HCrO 4 ) and divalent dichromate
2
(Cr 2 O 7 ) ions in the pH range of 2–6 and above pH 6; the dominant species is chro-
2
mate (CrO 4 ) ions [50]. The higher removal efficiency in the acidic pH (2–6) range is
Magnetic
PPy/F 3 O 4
Nano- separation Cr(VI)free
composites water
Magnetic bar
200 ppm Cr(VI)solution Nanocomposites +Cr(VI)Solution 0 ppm Cr(VI)solution
Fig. 21.10 Magnetic separation of PPy/Fe 3 O 4 nanocomposite after Cr(VI) removal.
Reproduced with the permission from Copyright 2011 Elsevier.