Page 627 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 627
Polymer nanocomposites for water treatments 579
produced using UV/H 2 O 2 , UV/ozone, UV/Fenton reagent, and TiO 2 -based material is
revealed to be efficient. The degradation of dyes by Fenton reagent takes place as.
Fe 2+ (aq)+H 2 O 2 (aq)!Fe 3+ (aq)+ROS (aq)
Dye (aq)+ROS (aq)!decolorized product+O 2 or H 2 O
The electrochemical methods have been recognized as simple, smart, portable, and
environmentally friendly, since electrode itself acts as a reactant in the redox reaction.
O 2 dissolved in solution is known to be electroactive and can be reduced electrochem-
ically. In fact, oxygen reduction reaction (ORR) in aqueous solution is a multielectron
transfer reaction, and at carbon and gold electrodes, the ORR generates various ROS
species.
Farhana et al. [44] developed a process by simply bringing together the electro-
chemical ORR and treatment of wastewater by AOP to overcome some of the limi-
tation of existing methodologies. Glassy carbon cathode was found to be suitable for
continuous generation of ROS reversibly by consuming air (O 2 ) dissolved in dye solu-
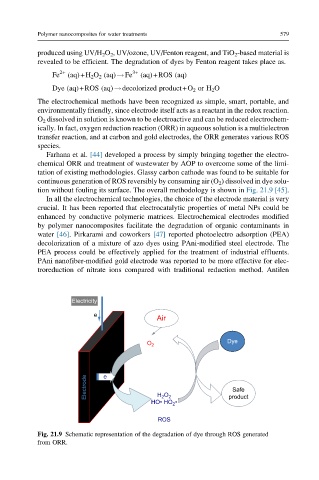
tion without fouling its surface. The overall methodology is shown in Fig. 21.9 [45].
In all the electrochemical technologies, the choice of the electrode material is very
crucial. It has been reported that electrocatalytic properties of metal NPs could be
enhanced by conductive polymeric matrices. Electrochemical electrodes modified
by polymer nanocomposites facilitate the degradation of organic contaminants in
water [46]. Pirkarami and coworkers [47] reported photoelectro adsorption (PEA)
decolorization of a mixture of azo dyes using PAni-modified steel electrode. The
PEA process could be effectively applied for the treatment of industrial effluents.
PAni nanofiber-modified gold electrode was reported to be more effective for elec-
troreduction of nitrate ions compared with traditional reduction method. Antilen
Electricity
e
Air
O 2 Dye
Electrode e Safe
O
2
H 2
HO• HO 2 • product
ROS
Fig. 21.9 Schematic representation of the degradation of dye through ROS generated
from ORR.