Page 634 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 634
Polymer nanocomposites for water treatments 585
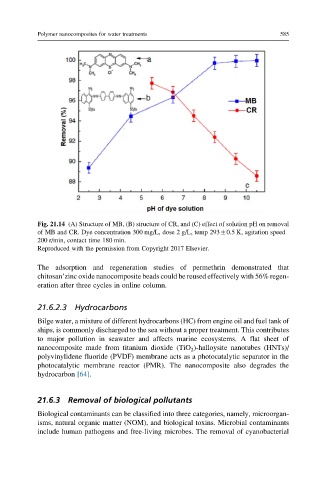
Fig. 21.14 (A) Structure of MB, (B) structure of CR, and (C) effect of solution pH on removal
of MB and CR. Dye concentration 300 mg/L, dose 2 g/L, temp 293 0.5 K, agitation speed
200 r/min, contact time 180 min.
Reproduced with the permission from Copyright 2017 Elsevier.
The adsorption and regeneration studies of permethrin demonstrated that
chitosan’zinc oxide nanocomposite beads could be reused effectively with 56% regen-
eration after three cycles in online column.
21.6.2.3 Hydrocarbons
Bilge water, a mixture of different hydrocarbons (HC) from engine oil and fuel tank of
ships, is commonly discharged to the sea without a proper treatment. This contributes
to major pollution in seawater and affects marine ecosystems. A flat sheet of
nanocomposite made from titanium dioxide (TiO 2 )-halloysite nanotubes (HNTs)/
polyvinylidene fluoride (PVDF) membrane acts as a photocatalytic separator in the
photocatalytic membrane reactor (PMR). The nanocomposite also degrades the
hydrocarbon [64].
21.6.3 Removal of biological pollutants
Biological contaminants can be classified into three categories, namely, microorgan-
isms, natural organic matter (NOM), and biological toxins. Microbial contaminants
include human pathogens and free-living microbes. The removal of cyanobacterial