Page 116 - Pressure Swing Adsorption
P. 116
L
90 PRESSURE SWING ADSORPTION PSA CYCLES: BASIC PRINCIPLES 91
Table 3.3. Effect of Pressure Ratio on Separation of Air on Umon
Carbide RS~IO Molecular Sieve~ "'
High Low Nitrogen Product "
pressure pressure Pres~ure recoverv purity
(kPa) (kPa) ra110 (%) (mol % N ) Productivitv " <O
•
2
:;
200 113 1.77 41.56 82.3 4.78 0 ,o
0
358 123 2.91 22.46 93,7 3.9 ~
<
620 154 4.03 12.54 98.2 2.61 ! , ,o
200 113 i.77 13.83 93.8 1.00 '' <
358 123 2.91 22.46 93.7 3.9 ,0
620 152 4.08 23.05 93.7 5.46
0
0 <O
High-pressure adsorptmn = 35 s, blowdown = 2 s. purge= 3 s. pressunzat1on = 15 s. Purge volume "' "' '"' '"
=0.0\66x IO-·• m~ at STP.
Source: Ref. 19 /a)
Table 3.4. Effect of Purge Pressure on Separatlon of Air on Union
Carbide RS-IO Molecular Sieve""
High pressure
(kPa) 200 358 620
Low pres1mre 113 Ml 123 78 152 119
(kPa)
Nitrogen recovery 13.83 16.87 22.46 21.83 23.05 23.12
(%)
, ProducI purity 93.8 93.8 93.7 93.7 93.7 93.7
(mole% N )
2
High-pressure adsorpuon = 35 s. hlowdown = 2 s, purge= 3 s, press1mza11on = 15 s. Purge volume
... n.01oox w·· • in·' STP.
Su11rce: Rel. 19. 0 16 2,
Tlitt'.' '""'
/b)
recovery 1s decreased. For the same purity_ both recovery and productivity
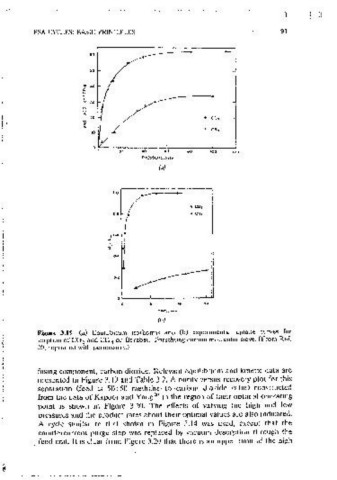
increase with rncreasing feed pressure. The recovery gam ts relatively less m Figure 3.19 (a) Equilibrium isotherms and (b) exoenmental u01ake curves for
sorpt1on of CO and CH on Bergbau~Forschung carbon molecuiar sieve. (From Ref.
the higher-oressure region, but the mcrease in productivity is consistent. 2 4
20; reprmted with perm1ss10n.)
Moreover, subatmosphenc blowdown and purge are advantageous (in terms
of recovery) at low feed oressures, but the advantage disappears as the feed
pressure is mcreased. The latter finding further confirms that in a Skarstrom fusmg component, carbon dioxide. Relevant equilibrium and kinetic data are
cvclc hlowdown loss becomes dommant at hig11 feed pressure. Product punty presented in Figure 3.19 and Table 3.2. A purity~versus~recovcry plot for this
may therefore l)C controlled hy either the feed oressure or the purge rate or separation (feed 1s 50: 50 methane-to-carbon dioxide ratio) construct.c<J
by a combination of both of these. from the data of Kapoor and Yang 20 in the region of thclf oomnal ooeratmg
pomt is shown m Figure 3.20. The effects of varvmg the high and low
oressures and the product rates about their optimal values are also indicated.
3.4.3 Equilibriu•m and Kinetic Effects Reinforce
A cycle similar to that shown m Figure 3.14 was used, exceot that the
Scparatwn of methane from methane-carbon dioxide mixture on :, carhnn countercurrent purge step was rcpiaced hy vacuum. desorption through the
molecular sieve ts an example where equilibrium is 1n favor of the faster~dif~ feed end. It is clear from Figure 3.20 that there 1s an upper iim1t of the high