Page 118 - Pressure Swing Adsorption
P. 118
I ! iii
92 PRESSURE SWING ADSORPTION PSA CYCLES: BASIC PRINCIPLES 93
100
1001 P., (atm7
1
v 2 □
u □~\ ..::::::----:0' -□ v Q
0 90~ u ' 3
u u 0 95 ~
0 6 0
C 0 '
Q \o ,, ii ~
s BO C ~•
w ' 2
C I C
w
0 0,
"' 0 90~ \ ~',,0~5
v
• 70 0 C
v
e
C
N ",\,~
• 0 ~ (2. 36-3. 72 atm) N
0 60 ~
,: 0 P.. m. 33- 1 atm) 0
□ Product rote ,: ·t
(0, 21-1. 35 l i trQs STP/cycle)
500
20 40 60 80 100 s9io 40 50 60
.?.: recovrery of methane ¼ recovery of nitrogen
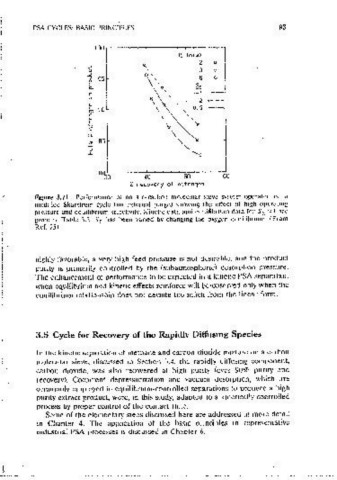
Figure 3.20 Effects of feed pressure, desorption pressure, and product rate on the Figure 3.21 Performance of an <Hr-carbon moiecuiar sieve svstcm operated on a
cxpcnmcnlal puntv and recovery of Cll, 1 from CH /C0 2 scparai1on by pressure modified Skarstrom cycle (no external purge) showing the effect of high openiting
4
swing ;.idsorpt1on on a carbon molecular sieve. The arrows indicate the direction of pressure and equilibrium se1cct1v1tv. Kinetic data and cquillibnum data for S 1 •. = 1 are
mcrcasmg parameter values. (Data taken from Ref. 20.) given m Table 3.2. SE has been vaned bv changing th·e oxygen equilibrium. (From
Ref. 25)
ooerat10g pressure beyond which both purity and recovery decline. With
highly favorable, a very high feed pressure 1s not desirable, anct the product
atmospheric blowdown the maximum methane oroduct purity that can be
punty 1s primarily controlled by the (suhatmospheric) desorption pressure.
achieved by raising the adsorption pressure is therefore limited to about
The enhancement of performance to be expected in a kinetic PSA separation
70%. Further improvement m product ounty 1s essentrnlly controlled by the when equilibrium and kinetic effects remforce will be 'Observed only When the
(subatmosohenc) desorption pressure.
eauilibnum relat1onshio does not deviate too much from the linear fonn.
These observatmns provide an mteresting contrast with similar perfor-
mance profiles for the kinetic air separation process (Figure 3.21). In Figure
3.21 all profiles are monotonic with no evidence of an upper limit. While
there must always be a theorct1cal limit for the high operating pressure,
beyond which both recovery and punty decline, Jt is clear that this limit lies 3.5 Cycle for Recovery of the Rapidly Diffusing Species
well beyond the normal range of operating pressures for air separat10n on r;
carbon molecular sieve and therefore does not limit the system -performance. In the kinetic separation of methane and carbon dioxide mixture on a carbon
In contrast to the methane-carbon dioxide system, a high-punty nitrogen molecular sieve, discussed m Section 3.4, the rapidly diffusing component,
raffinate oroctuct can therefore be achieved simoly by raising the adsorollon carbon dioxide, was also recovered at high ounty (over 90% purity and
pressure, without recourse to subatmosphenc desorption. recovery). Cocurrent depressunzation and vacuum desorption, which are
The key difference between a,r separation and ~ethane-carbon dioxide commonly employed in equilibrium-controlled separations to produce a high
systems appears to lie m the shape ot the equilibrium isotherm for the more punty extract product, were, in this study, adapted to a kinetically controlled
strongly adsorbed soecies. For nitrogen-oxygen on CMS the isotherms are of process by proper control of the contact time.
linear or slightly favorable (type I) form, whereas, m the relevant pressure Some of the eicmentary steos discussed here are addressed in more detail
range, the isotherm for carbon dioxide on CMS is highly favorable, appr()ach- tn Chanter 4, The appiication of the 11as1c orinCiples m representative
ing the rectangular limit. If the isotherm for the fostcr~diffusing species 1s industnai PSA oroccsses ts discussed in Chanter 6.