Page 127 - Pressure Swing Adsorption
P. 127
\I,
102 PRESSURE SWING ADSORPTION EQUILIBRIUM THEORY 103
i.O --.,..-
' ' ' column isotherm 1s
-
c; _ y,P (- l - s f, RT)
n;- RT l+-,---r,P·
0.8 f- -
z In terms of wave movements, this 1s the cumulative amount of adsorbate ,
0 f- -
;::
u admitted at P and y, to an m1tially clean adsorbent (i.e., totally evacuated or
~ 0.6 f- OECREASING - prepressurized with a less Slrongly adsorbed component), stopping at l)rcak•
"'
~
f-
w ' FLOW RATE - through. The column isotherm is discussed later m other contexts.
J The parameters f3 and 0 are related to velocities via Eqs. 4.5, 4.9, and
0 0.4 -
" f- 4.10. Therefore, it 1s possible to measure thetr effective vaiues in break-
N
z through exoer1ments. In one type of exoeriment, a fixed bed of adsorbent ts
~ -
initially ourged and pressurized with the light component. The feed 1s then
0.2 f- admitted to the bed at the same pressure. BY simply momtormg the influent
and effluent flow rates, the value of 0 (or f3 when both isotherms are
-
f-
oracl!cally linear) may be found from:
0.0 ' ' i ' ' '
0 60 1 20 180 240 300 360 420 480 - Qoo,/Q;olf-
( 4.11)
TIME (SEC)
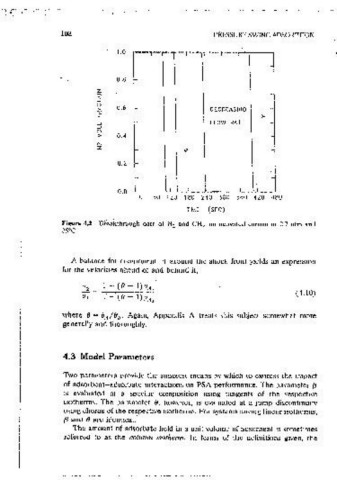
Figure 4.2 Bl'eaKthf"ough data of N and CH 4 on activated carbon at 2.7 atm and ( = f3 for linear isotherms)
2
25°C.
where the product is pure (i.e., YA" = 0), the bed pressure is kept constant,
AP= 0, and the volumetnc flow rates, Qs, are constant. In an alternative
type of expenment the adsorbent bed is mltially equilibrated with feed. Then
A balance for comoonent A around the shock front yields an expression the pure heavy component is admitted to the bed a:t the same oressure. By
for the velocities ahead of and behind it, s1moly monitoring the influent and effluent flow rates, the vaiue of 0 (or {3)
may be found from:
v = 1 + ( e - 1) y A,
2
v, 1+(0-l)YA, ( 4.10)
where O = OA/0 • Agam, Appendix A treats this subject somewhat more
8 ( = /3 for linear isotherms) ( 4.12)
generally and thoroughly.
Note that when both isotherms are linear, the values obtained m the two
types of experiments should be consistent, but if isotherm curvature 1s
significant, the values of fJ detennmed m the two types of exoenments may
4.3 Model Parameters be different. In that case, the first type of measurement would be more useful
when the light component is des1red, and the second type would be more
Two parameters provide the s1molest means by which to express the imoact useful when the heavy component 1s desired.
of adsorbent-adsorbate interactions on PSA performance. The parameter /3 This approach has several advantages: a range of different operating
1s evaluated at a specific composition using tangents of the respective conditions (feed comoosit1on, pressures, and cycle times) can be examined,
isotherms. The parameter 0, however, 1s evaluated at a Jump discontmu1ty the effects of mmor vanations m packing and_/or adsorbent prop!!rt1es can be
using chords of the respective isotherms. For systems havmg linear isotherms, assessed directly, and even effects of dispersion and' diffusion can be identi•
{3 and 8 are identical. fled and easily resolved.
The amount of adsorbate held in a unit volume of adsorbent is sometimes As mentioned earlier, examples of exoenmental breakthrough .curves, for
referred to as the column isotherm. In terms of the definitions given, the methane (A)-mtrogen (B) on activated carbon, are Shown in Figure 4.2. The