Page 157 - Pressure Swing Adsorption
P. 157
.:1
132 PRESSURE SWING ADSORPTION EQUILIBRIUM THEORY 133
4.5 Experimental Validation
t.O
a.a The beauty of local eauilibnum theones lies m their s1molic1ty and the1r
ability to draw attention to the most 1moortant ooeratmg conditions, geomet-
Q.6
R ric oarameters, and ohys1ca1 oropert1es. Unless these predictions agree with
0.4 reality, however, that beauty 1s superfluous. This section reviews some of the
0.2 experimental work that has been deliberately aimed at verifying local equilib-
rium theones.
The early expenmental studies appear to have been conducted as "black-
box" studies, in which cycling freauency, feect-to~ourge flow rate ratios,
pressures, etc. were vaned systematically. As these parameters were manipu-
0
lated, the performance (flow rates, product and byproduct purities) and other
,20
variables were monitored. Compansons with theory were made retrospec-
tively. Perhaos the first comparison of this sort was that of Mitchell and
/c)
Shenctalman. 24 The!T application was the removal of 1 % :CO from a helium
2
earner using silica gel. They did not find close corre·spondence with their
0.10
eauilibrmm theory; so they mtroduced a mass transfer resistance to account
0.08 for the discrepancy. This modification allowed them to bracket the observed
o.06 behavior, but neither model was accurate over the enttre ,range of conditions.
R Flores-Fernandez and Kenney 25 developed a more broadly applicable equ1-
0.04
libnum theory, and solved it via finite differences and a commercial package
0.02
I oxygen from air usmg 5A zeolite, and obtamed fatr agreement: within 15%
known as CSMP. They tested t.heIT modei bv exveriinentally separatmg
l for the prediction of feed flows, and within 12% for the prediction of
recovery.
0 f More recently a different approach has been taken, which is to build an
/ 4 3 expenmental system m such a way that the mherent iassumptions of the
0/o Dead \lo\ome ' equilibrium theorv are closely approached, then to operate it m such a wav
I
I advantage of exammmg condit10ns that are of most practical interest, as well
/d) that the best possible performance is expected. This: approach has the
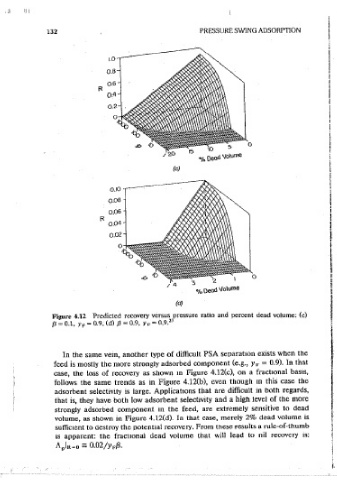
Figure 4.12 Predicted recovery versus pressure ratio and percent dead volume: (c) as usmg the theory as a tool to guide the experiments. Several different cycles
/J = 0.1. Ye= 0.9. (d) /J = 0.9. Ye= 0.9. 23 ' have been evaluated this way, but to conserve space only three are discussed
feed is mostly the more strongly adsorbed component (e.g., YF = 0.9). In that I four-step cycle with combined feed and cocurrent blowdOwn, and a five-step
here. They are: the four-step cycie emoloying pressunzation with product, a
cycle incorporating a rmse step m order to obtain two ;pure products. The
In the same vem, another type of difficult PSA separation exists when the
underiymg theory of all these cycles was discussed earlier In this chaoter.
follows the same trends as m Figure 4.12(b), even thOugh m this case the l Section 4.4.1, which applies for the four-step pressunzatton-with-product
The first test determmed the vaiidity of the theory that was described m
case, the loss of recovery as shown m Figure 4.12(c), on a fractional basis.
adsorbent selectivity is large. Applications that are difficult in both regards, cycle, shown m Figure 4.1. The experimental system was a two-bed apparatus
that is, they have both low adsorbent selecttv1ty and a high level of the more I contammg zeolite SA, designed to separate oxygen from dry atr (Kayser and
strongly adsorbed component m the feed, are extremely sensitive to dead KnaebeI 20 ). The temperature and pressures were such that nearly linear
volume, as shown in Figure 4.12(d). In that case, mereiy 2% dead volume ls isotherms were expected, and the eou1pment was designed so that the
sufficient to destroy the potential recovery. From these results a rule-of-thumb ' assumptions cited in Section 4.1 were valid (including mihtmal dead volume).
is appat'cnt: the fractmnal dead voiume that will lead to nil recovery 1s: Six sets of experimental conditions were tested, and for each, the system was
AplR-o = 0.02/Ye/3- operated until cyclic steady state was achieved. The pressure ratm range was
;
i