Page 161 - Pressure Swing Adsorption
P. 161
li !
136 PRESSURE SWING ADSORPTION EQUILIBRIUM THEORY 137
1.0 100
X=FRACTION PURGE N ' '-+I -.;1 N- N--'-
95 - ,, 0 -
0.8 - --€> -
>- au- 0
"' 90 - -- --- ---
GAS
SYMBOL
w
SOURCE
>
Collin"
8 0.6 0 N Oxygen Collins
Nitrogen
w " 0 Oxygen Sircor
Sircar
Nifrogeri
"' >- ,_ BS - I " -
z 4-STEP PSA oc
w
C) "'
n.
>-
x ~
0 80 - -
0.2
75 - -
1 0 1 0 40 50 60 70 80 90 100
0.0 1 0 0 1 2 70 ' ' I ' '
fp =PH/PL RECOVERY
/a) Figure 4.15 Recovenes and purities of oxygen and nitrogen:from air, comparing the
12
present five-step cycie with a similar cycle proposed by Sircar. 22
i.O
X=FRACTION PURGEO the Reynolds number m the bed. Finally, across the spectrum of ootentiai
PSA applicatwns the mtrus1on of heat effects can be expected to vary widely.
0.8 Such effects may be either benefictal or detrimental, as discussed m Sec-
tion 4.8.
>-
"'
w
>
0
u 0.6
w [ 4.6 Model Comparison
"'
z '
w This section examines the salient features of four distinct local eauilibnum-
C) I
0 0.4 basect models that have been developed over the past severai vears. In
,_
"'
z I general, it 1s fau to say that the obv10us differences between these models
are, remarkably, not in the final eouatmns. Rather the differences lie in the
0.2 allowable values of certain variables, which affect the parameters of the
I I models. The onginal derivations contamed subtle· assumptions that imposed
0.0 1 oo 1 0 1 1 0 2 tight restrictions on the parameters. Thus, 1t was not merely an oversight that
larger ranges of the parameters were not examined.
p t
fp = PH / .L 4.6.1 Four-Step PSA Cycie: Pressurization with Product
i The first and simplest model, that of Shendalman and Mitchell, can be
{b) 2
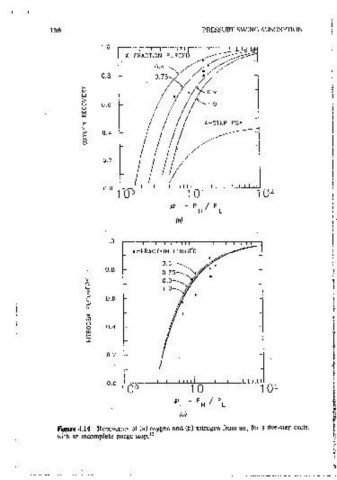
Figm·e 4.14 Recovenes of (a) oxygen and (b) mtrogen from air, for a five-step cycle, ·applied to the PSA cycle shown m Figure 4.1, and the resultmg eauallon for
with an incomplete purge step. 12 recovery can be expressed in the same form as Eq. 4.27. This model assumes
f that the more strongly adsorbed component 1s a trace contammant and that it
[
t