Page 165 - Pressure Swing Adsorption
P. 165
HI
!i I
140 PRESSURE SWING ADSORPTION EQUILIBRIUM THEORY 141
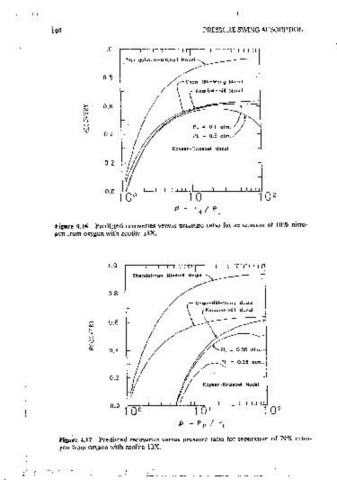
sorpt1on of oxygen), are m good agreement at low to moderate pressure
i .0
ratios. Even when curvature of the nitrogen isotherm- ts taken into account,
Shandalman-Mlkhell Model
the differences are mmor, as long as PL 1s small. When H ts raised to Just
0.25 atm, to correspond to the comoanson m Table 4.2, the effect of
0.8
Chon-HUI-Wong Model
curvature becomes oronouncet1 at high pressure ratios.
(' Knaebel-HIii ·Model For the more typical feed composition of 79% mtr:ogen and ·21 % oxygen.
shown in Figure 4.17, there is practically no agreement among the modeis.
>- 0.6
~ That figure clearly shows the magnitude of ctev1ations,cau"Sed by 1gnormg: (I)
w
> sorption of the light component (the difference between the Shenctalman-
0
u
w PL= 0.1 aim.// Mitchell model and that of Chan et al.), (2) compos1t1on deoendence of
~
0.4 PL = 0.5 aim,/ interstitial velocity (the difference between the model of Chan et al. and the
Knaebel-Hill model), (3) the effect of ,sothenn curvature (the difference
Kayser-Knoebel Model
between the model of Knaebel-Hill and the Kayser~Knaebel model), and
0.2 ! ' finally (4) the 1moact of absolute oressure when the 1sotherrns are not linear
(the difference between the values of PL within the Kayser-Knaebel model).
In all of these comoansons, recovery (at a given pressure ratio) is always
1 0 2 dimmished by taking mto account more of the effects mentioned.
4.6.2 Four-Step PSA Cycle: Pressurization with Feed
Figure 4.16 Predicted recoveries versus pressure ratio for separation of 10% nitro- The differences between the assumptions of the mode is of Chan et al.} and
gen from oxygen with zeolite 13X. 13
Knaebel and Hill are also evident in PSA cycles that empioy pressurization
i.O
o.O
Shendalmon-Mitchell Model Chan-Hill-Wong I.lode! --
Kna~bel-H!ll Model --
0.8 0.8
r Chon-Hill-Wong Model I = 0.1
\ f Knoebel-HIil Model
>- 0.6
>- 0.6
~ °'
w
w
> >
0
0 u
u w
w
~ PL = 0.05 aim. "' 0.4
0.4
fJ = 0.5
=
rL 0.25 otm.
0,2
0,2
I/ I Koym-Knoobot "•dot fJ = 0.9
0,0 1 oo 1 0 2
Figure 4.17 Predicted recoveries versus pressure ratio for separation of 79% nitro- Figure 4.18 Recovery versus fl for various adsorbent seiectivities, y 8 = 0.9, compar~
gen from oxygen with zeolite 13X. ing the models of Chan et al. and Knaebel and Hill. F ·