Page 164 - Pressure Swing Adsorption
P. 164
138 PRESSURE SWING ADSORPTION EQUILIBRIUM THEORY 139
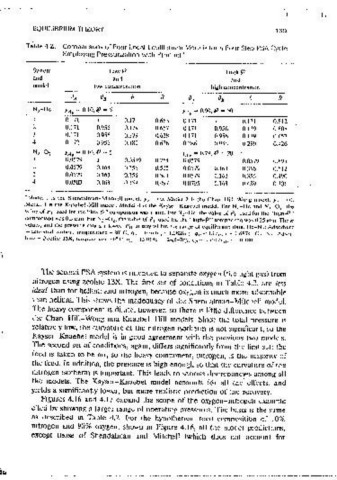
follows Henry'~ law, whik the carrier 1s not adsorbed. In terms of the Table 4.2. Comparisons of Four Local £quilibnurn Model.<\ for ;1 Four-Sien PSA C:y<:I,~
parameters used above, these are: YuF ~ i (i.e., Y,,i" --t- 0), { = I, fJ = fJ = Employing Pressunzation wiHl Product" '
BA,,= /3A, and /3 8 = 0 = l. The next eauilibrium model, developed by
8
Chan, Hill, and Wong? 1s iess restnct1ve, though 1t also assumes that the Svsiem LowP HighP
and and and
more strongly adsorbed component is very dilute. lt allows for adsorption of
model low concentrat10n high concentrauon
the carrier gas (following a linear isotherm). These restrictions amount to the
0 R 0 R
followmg: y = 1, { = i, and /3 = 0 = (3A /(3 . Both of these theones
8 F O 8 U
ignore the effects of uptake (and release) on the interstitial gas velocity. That NrHe YAF = 0.10, fJ = 5 YAF = 0.90, fiJ = 50
assumotion allows the cycle to be analyzed easily, but it leads to potentially 0.171 0.171 0.663 0.171 0.171 0.812
serious errors, because it implies that the molar flows of feed and oroduct are 2 0.171 0.956 0.179 0,657 0,171 0.956 0.179 0.805
3 0.171 0.956 0.179 0.638 0.171
identical, and similarly that the amount of gas required for pressunzation 1s 0.956 0.179 0.657
4 0.172 0.956 0.180 0.636 0.286 0.956 0.299 0.426
equal to the amount exhausted during blowdown.
A similar model, suggested by Knaebei and Hill, 13 mcoroorated adsorp- N2-02 YAF=O.IO,fJ,,,5 }'AF= 0.79. fJ = 20
0.0579 l 0.0579 0.754 0.0579 0.0579 0.895
tion of both components of an arbitrary binary mIXture ri.e., Yn, E (0, 1 )1. '
2 0.0579 0.161 0.356 0.515 0.0579 0.163 0.356 0.612
Thus, the vanat1on of velocity arising from comoosition vanat10ns was taken c 0.0579 0.163 0.356 0.501 0.0579 0.163 0.356 0.490
mto account. Adsorption equilibrium, however, was still restricted to linear 4 0.0583 0.163 0.359 0.497 0.0795 0.163 0.489 0.301
isotherms. Hence, the parameters of that model are: { = 1, (3 = 0 = f3 .. ,,lf3 ,,,
11
and /3;,, = 0;. Finally, the model of Kayser and Knaebel,' which 1s !he basis of "Model i rn the Shendalman--Mitchc!I model, yA ··•O. Mndd 2 is the Cl1<w--Hill-Wong m1,dcl, Y,-1,. --.0.
1
,Model 3 is the Knaebel-Hill model. Model 4 is the Kayser-Knaebel model. for N,-He and N,-o·,, rhe
Eqs. 4.1-4.27, allows for nonlinear isotherms, as well as arbitrary comoos1-
value of PL used for the "low-P" companson was I aim.· For N -He, 1he vaiue of PL used for the "high-V ..
2
tton. Thus, m that model the parameters are distmct, and the followmg companson was 0.5 atm. For N 2 -0 2 , the value of PL used for the "high-.?'" comparison was 0.25 aim. These
combinations are allowed: t· ::P I, /3 * 0 * {3A * {3A, and /3n * 8 0 (although values, and the pressure nmos, allowed P 11 to sruy within the range ol equilihrmm data. He-N,: /\d~orheni
0
the last mequality is dropped in the exarnpies to follow). - acllv1,1ed carbon, \Cmperalurc ~ 2n"C, qA,... 1fl.01c.-1 ·- 12,H2f,.·.~. q 11 =0,ilc,,, ~ .. , 0.6H74 (), N,: ,\d~or,
bent= Zeolite J3X, temperature.., o~ C, qA = 15.011c.-i - 24,547d, q 11 = 4.1542c 11 , ~- = 0.4kfJ
If we use the result of the most comoiete ctenvatIOn to predict recovery,
viz., Ea. 4.27, the differences between the models are reflected in the
allowable values of the parameters. To emphasize the impact of the different
parameters on the four models, two different adsorbent-adsorbate systems, The second PSA system ,s intended to separate oxygen (the light gas) from
which are both simulated at two different sets of conditions, will be discussed nitrogen usmg zeolite 13X. The first set of conditions m Table 4.2, are less
m the followmg paragraphs. ideal than for helium and nitrogen, because oxygen is much more adsorbable
In the first PSA system. a very light gas, helium. 1s to be removed from than helium. This shows the madeauacy of the Shendalman-Mitchell model.
nitrogen (cf. Figures 4.2 and 4.3). Nitrogen 1s much more adsorbable than The heavy component is dilute, however, so there ts littie difference between
helium, but not to the oomt that the isotherm is very nonlinear (at or below the Chan-Hill-Wong anct Knaebel-Hill models. Since the total pressure ,s
2 atm). The first set of conditions represents what might be thought of as an relatively low, the curvature of the mtrogen isotherm is· not significant, so the
ideal PSA application, since the light gas is taken as the maJor component. It Kayser-Knaebel model is in good agreement with the previous two models.
is perhaps not surprising that there 1s excellent agreement (i.e., within about The second set of conditions, again, differs significantly from the first set: the
3%) among the Shendalman-Mitchell, Chan et ai., Knaebel-Hill, and feed ts taken to be a1r, so the heavy component, nitrogen, 1s the majonty of
Kayser-Knaebel models for that situation, and that the oredicted recovery ts the feed. In addition, the pressure is high enough so that the cmvature of the
high. The second set of conditions involves a significant shift: now the heavy nitrogen isotherm is important. This ieads to serious discrepancies among all
component is the maJor comoonent of the feed, and the pressure is high the models. The Kayser-Knaebel model accounts for all the effects, and
enough so that curvature of its isotherm ts important. As a result, the two yields a significantly Jower, but more realistic oredictidn of the recovery.
simplest models agree, but they must be incorrect smce the heavy component Figures 4.16 and 4.17 exoand the scope of the oxygen-nitrogen example
is not merely a trace contaminant. The Knaebel-Hill model accounts cor- cited by showmg a larger range of ooeratmg pressures. The basis 1s the same
rectly for comoositlon, yielding a 15% reduction of recovery. Correcting for as described in Table 4.2. For the hypotheticai feed composition of 10%
curvature of the nttrogen isotherm, vta the Kayser-Knaebel modei, reduces mtrogen and 90% oxygen, shown m Figure 4.16, all the model predicuons,
the recovery again by 23%. except those of Shendaiman and Mitchell (which does not account for