Page 230 - Pressure Swing Adsorption
P. 230
,l
206 PRESSURE SWING ADSORPTION
DYNAMIC MODELING OF A PSA SYSTEM
207
10 60
Experimental X .. Experimental X
Transient modg1 0 Transient modg:l
CCF modol V CCF modg;i 0 60
V
8 ... _ ' \
" ..,_ C \
0
•
:,
" ..,, 40 0
0,
0
L L
o._ ....
6
C .. C 40 C
C I "- 0 \ \ \ "-
• ' ' :,._ \ 0
0,
.. 4 ' - -s ____ - .Iii L •
:,._
X
0
L
0
>
>
0
0
0
• 20 0 0 L 5 •
0 L
:,: N 20 ><
r
2
r
r
0'--~-J-~--'--~--'--~-'o o L~--'--~J--2::=t:::!~_J o
15 20 25 30 35
0 . 5 I. 5 2
L/v ratio (s)
OH G
(a) ( b)
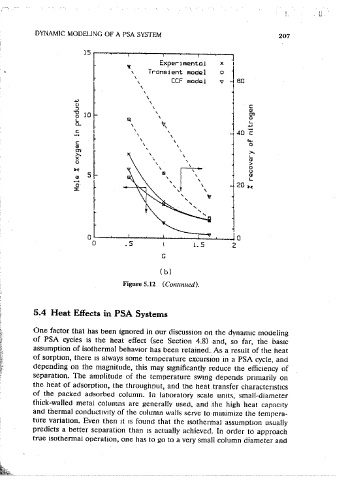
Figure 5.12 (Contmued).
Figure 5.12 Effects of (a) L/ v H ratio (G = 1.0) and (b) purge to feed velocity ratio
0
( L / v 0 u = 25 s) on the purity and recovery of nitrogen product m a PSA air
scoarauon process (Skarstrom cycle) showmg comoanson among the expenmental
reSults, the CCF modei, and the transient model predictions. Adsorption pressure = 3
atm, blowdown/purge pressure= 1.0 atm, adsorption/desorption time= 60 s, pres- 5.4 Heat Effects in PSA Systems
sur1zati'on/blowdown time= JS s, diffusional ttme constants used for oXygen and
nitrogen were 3.73x10- 3 s- 1 and 1.17x10- 4 s-', respectively. The CCF model One factor that has been ignored in our discussion on the dvnam1c modeling
results were comouteci with n = 15 for both oxygen and nitrogen; cycle-time-denen- of PSA cycles 1s the heat effect (see Section 4.8) and, so far, the basic
dent !1 values according to the correlation of Nakao and Suzuki were used in the assumption of isothermal behavior has been retained .. As a result of the heat
transient model simulation . (From Ref. 59.)
of sorption, there 1s always some temperature excursion in a PSA cycle, and
depending on the magnitude, this may s1gnificantiy reduce the efficiency of
separation. The amplitude of the temperature swmg depends primarily on
orinc101e to both kinetic and equilibrium-based separations. lts applicability the heat of adsorption, the throughout, and the heat. transfer charactenst1cs
to the latter class 1s restncted to trace systems with significant mass transfer of the packed adsorbed column. In laboratory scale units, small-diameter
resistance. The fact that one solid':'phase concentrat10n profile cannot be in thick-walled metal columns are generally used, and ,the high heat capacity
equilibnum with two different gas-ohase profiles precludes the use of this and thermal conduct1v1ty of the column walls serve to m1niml2e the tempera-
modei for eauilibrium-controlled separations with negligible mass transfer ture variation. Even then 1t 1s found that the isothermal assumot1on usually
resistance. Similar reasoning also precludes the extension of the CCF model predicts a better separation than 1s actually achieved. In order to approach
to account for heat effects. true isothermal ooeration, one has to go to a very small column diameter and