Page 234 - Pressure Swing Adsorption
P. 234
II
210 PRESSURE SWING ADSORPTION DYNAMIC MODELING OF A PSA SYSTEM 211
Table 5.1 J. Paramelers Used in Comouting the Theoretical Curves m the gas concentration according to tl1e ideal gas; law. The temperature
Figures 5.14 - 5.16 Showing the Existence of Two vanation in the bed at any given point was 20-40°C. The exoeiiments were
Different Cyclic Steady States
carried out in small-diameter columns (4.1 cm i.d.) and therefore suffered
SA zeolite;i Activated alumma,. from heat loss by wall conduct10n and exchange with the surroundings.
(Under true adiabatic conditions the temperature variat10n could reach
Feed gas composition 1 % Ethviene m 0.39% Moisture m I00°C.) In some studies they replaced the LDF rate equation with more
helium .., detailed diffusion models. In the mtroctuctory sect10n of this chapter it was
Particle diameter (mm) 0.7 4-5
Bed length (cm) 35.0 100.0 oOmted out that m an eouilibrium-controlled separation the detailed form of
Bed radius, i.d. (cm) 1.75 !0.0 the kinetic model 1s of only secondary importance. This 1s also true in the
o.d. (cm) 2.1
B_ed voidage 0.4 0.4
Feed temperature (K) 298.0 (ambient) 303.0 (ambient) I I
1 4 I I
G11s density (g/cm· ) 1.5 X 10- (1 atm) 1.2 X 10-·-• (1 a1m)
Adsorbt:nt densttY (g/cm·') 1.14' 1.2
Heal of adsorption (cul/mol) -8000.0J - 12404.0 I ---+--
Gas heat capaci1v (caljg °C) 1.2376 0.238
Adsorbent heat
capacnv (caljg "C) 0.206" 0.3
Heat transfer coefficient
2
(cal/cm s °C) 0.0 0.0 B
Effoct1ve ·axial thermal Deduced from
conductivity of fluid analogy of mass
(cal/cnt s °C) and heat transfer 0.0018
Adsorpl!On pressure (atm) 3.0 5.0
B\owdown/purge
pressure (atm) 1.36 1.0 10
6
Peclet number 110" 10 (plug flow) o,1-.)_ __ _j ____ -J-_ji::=:::::::_
L/VoH ratio (s) 5.91 4.0 C
Purge to foed veloc1tv ratio 1.54 2.0
f/o/co 837.W 8993.16 '
ffo/q, 0.92" 0.0 (linear) 2 -----
/
LDP mass transfer l1v,---v
1
coefficient (s- ) I II III
high pressure 0.19" 2.78 X 10- 4 ol-1-----+---------,---t-----;
low pressure O.IW l.3Q X I 0-· _,
Adsorption/purge time (s) 80.0 540.0
Pressunzat,on/blowdown I I
time (s} 20.0 140.0
, I
10
Heat capucuv and thermnl ccmductivity of steel were used to account for the wall effec1.
" C-hiharn and Su:i:uki. 4 ·
Ru1hven et al.'" 4 0 ' 10
J Farooq and Ruthven."] TIME. ■ 11
10
Hassan et al.
( a)
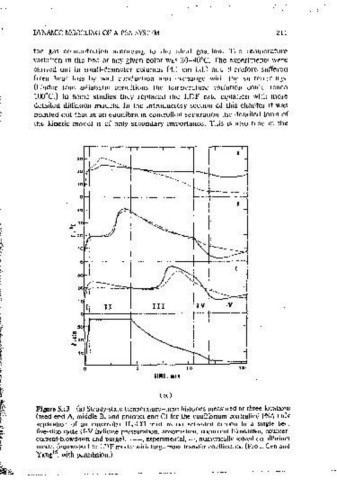
time. Convergence to cyclic steady state may be very slow under adiabatic Figure 5.13 (a) Steady-state temperature-tune histones measured at three locations
conditions, reqmrmg m some cases up to 100 cycles. A more detailed account (feed end A, middle B, and product end C) for the equilibrium controlled PSA bulk
of nomsothermal PSA simulation is given m Ref. 19. separation of an eammoiar H -CO mixture on activated carbon m a smgle bed,
2
five-step cycle (I-Vindicate pressurat1on, adsorpat1on, cocurrent blowdown, counter-
Yang and co-workers used s1inilar nonisothermai models to study several current blowdown and ourge). --, experimental,---, numencallv solved equilibrium
eauilibnum-controlled separation processes (see Table 5.1). They neglected model (eqmvalent to LDF model with large mass transfer coefficients. (From Cen and
the axial thermal conctuct1on but considerect the temperature ctepenctence of Yang 15 , with permissioo.)