Page 236 - Pressure Swing Adsorption
P. 236
l.ll
212 PRESSURE SWING ADSORPTION DYNAMIC MODELING OF A PSA SYSTEM 213
sor---r--,--..,.--~--,--,-----,c--.--,--....---.----,---,---. { 310,-----,----,---.----~-------7
A
p I I II III IV
~ JO I 1 _jl _____ - I fil
- ' ' - '
~-,
I , I I 305 7.-.---'
JO~, I 7 f
. I
300
50 B
p ' ,, ------
/ r-- - ' I •
I
" ' ,,
I I 295
JO I I ..........
' ' ' '
I
I ' ' ' '
101---.-------;------,---- ' •
•
so C 290 :ariiblent temp.
' 5 2 :f"eed temp.
3 :pre-bed (a.1umina)
,,~-------------..1. __ :20 cm from 1n1et
~ JO i ' ' ' •
I ,- ' 285 5 :40 cm from inJet
' ' ,,
--- 6 : 60 cm from 1 nl et
.j 7 :80 cro. fro.1¥l inlet
0 12tl 240 360 :nm!1-~-,..20s--'---,;-;0r--,sh0.--"a"0,l--f1•a0 0--f1:0 20s----.-,.:..0r-,t'is"0.----!1 a0
IIME.1
(b)
TIME, S
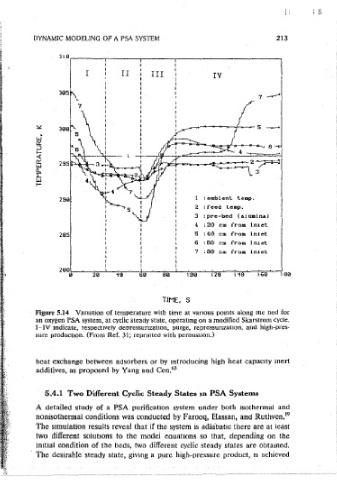
Figure 5.13 (b) Steady-State temperature histones for an eouilibru1m PSA bulk Figure 5.14 Variation of temperature with time at vanous oomts along the bed for
separtion (eQuimolar mixture of H rCH on activated carbon,---, expenmental; --. an oxygen PSA system, at cyclic steady state, operating on a ;modified Skarstrom cycle.
4
1
macrooore diffusion model), Other details as m part (a). (From Doong and Yang \ I-IV indicate, respectively deoressunzation, purge, repressurization, and high-pres~
with oermiss,on.) sure production. (From Ref. 31; reprmted with oerm1ss1on.)
prediction of nomsothennai behavior. Representative temperature profiles heat exchange between actsorbers or by mtrodudng high heat capacity inert
from the work of Yang et al. are shown in Figure 5.13. Since direct additives, as proposed by Yang and Cen. 63
measurement of concentration profiles in the bed ts not easy, the tempera-
ture vs. time profiles measured at various positions in the column provide a
5.4.1 Two Different Cyclic Steady States m PSA Systems
oractically useful way of Iocatmg the advancing mass transfer zone in the
column. Esp1tr.lier-Noel 31 observed a sharp nse m the bed temperature at A detailed study of a PSA purification system under both isothermal and
the product end pnor to breakthrough of mtrogen durmg the high pressure nomsothermal conditions was conducted by Farooq, li!assan, and Ruthven. 19
production step of a PSA air separation cycle for oxygen production on 5A The simulation results reveal that if the system ls adiabatic there are at least
zeo!ite. The temperature vanation at vanous pomts along the bed is shown m two different solut10ns to the model equations so that, depending on the
Figure 5.14. The temoerature profiles are also useful for understanding the imtial condition of the beds, two different cyclic steady states are obtamed.
1mprovect oerformance of a PSA seoaration that may be achieved by allowmg The desirable steady state, g1vmg a pure high-oressure product, 1s achieved