Page 239 - Pressure Swing Adsorption
P. 239
< 11
216 PRESSURE SWING ADSORPTION DYNAMIC MODELING OF A PSA SYSTEM 217
10 - - I attemot to restart the system after It had been standing m a loaded condiuon
THEORETICAL THEORET J CAL
Adsorption Adaorptlon -- for some time could well lead to the saturated bed Steady-state operation
Oli!sorption O•soro<,on ---1 rather than the more desirable clean bed steady-state operation, which gives
EXPERIMENTAL EXPERIMENTAL
Adsorption s Adsorpt I on " a purer product. Of course the change of steady state would not show m the
Qgsorpt1on product concentration if the system is operated such that the concentration
O,u,;orpt I on "'
front is mamtamed well inside the column with a large margin of safety.
G G
m ,. ______
•
3 0
i • References
. -s 1. J.C. Kavser and K. S. Knaebel, Chem. Eng. Sci. 440), l (1989).
2. G. Flores-Fernandez and C. N. Kenney, Chem. En,:. Sci. 38(6), 827 (1983).
J. J.E. Mitchell and L. H. Shendalman, A/Ch£ Symp. Ser. 69(134), 25 (1973).
t 4. K. Chihara and M. Suzuki, J. Chem. Eng. Japan 16(1), 53 (1983).
-10 ~--.~.--."-,--.J ..• -~-.L.--J -100=---.~.--.. _,,---.J .• --~ .. '-,---'
0 I 5. K. Chihara and M. Suzuki, J. Chem. Errg. Japa,1 J6(4), 293 (1983).
Z/L ZIL
6. J. W. Carter and M. L. Wyszynski, Chem. Eng. Soc. 38(7), 1093 (1983).
<al {bl
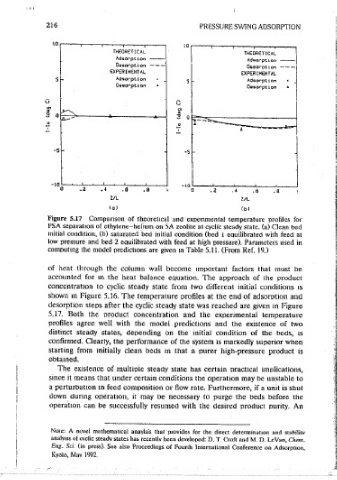
Figure 5.17 Comparison of theoretical and experimental temperature profiles for 7. N. S. Raghavan, M. M. Hassan, and D. M. Ruthven, AlChE J. 31(3), 385 (1895).
PSA separation of ethylen·e-hdium on SA zeolite at cyclic steady state. (a) Clean bed R P. L. Cen, W. N. Chen, and R. T. Yang, Jrrd. Eng. Chem. Process Des. Dee. 24(4), 1201
initial condition, (b) saturated bed initial condition (bed 1 eauilibrated with feed at (1985).
low pressure and bed 2 eouilibrated with feed at high pressure). Parameters used in
computing the model oredict1ons are given m Table 5.11. (From Ref. 19.) 9, R. T. Yang and S. j_ Doong, A/Ch£ J. 31(11), 1829 (1985).
10. M. M. Hassan, N. S. Raghavan, D. M. Ruthven, and H. A. Boniface, A/Ch£ J. 31(12), 2008
of heat through the column wall become important factors that must be (1985).
accounted for m the heat balance equation. The approach of the product 11. N. S. Raghavan and D. M. Ruthven, A/Ch£ 1. 3102), 2017 (1985).
concentration to cyclic steady state from two different initial conditions 1s
12. S. J. Doong and R. T. Yang, A/Ch£ J. 32(3), 397 (1986).
shown m Figure 5.16. The temperature profiles at the end of adsorpl!on and
desorption steps after the cyclic steady state was reached are given m Figure 13. M. M. Hassan, D. M. Ruthven, and N. S. Raghavan, Chem. E11g. Sci. 41(5), 1333 0986).
5.17. Both the oroctuct concentration and the experimental temperature 14. N. S. Raghavan, M. M. Hassan, and D. M. Rulhven, Chem. Eng. Set.41(11), 2787 <1986).
profiles agree well with the model predictions and the existence of two
15. P. L. Cea and R. T. Yang, Ind. Eng, Chem. Fundam. 25(4), 758 (1986).
distinct steady states, depending on the initial condition of the beds, 1s
confirmed. Clearly, the performance of the system 1s markedly supenor when 16. H. S. Shin and K. S. Knaebel, A/ChE I. 33, 654 (1987).
starting from initially clean beds m that a purer high-pressure product is 17. S. J. Doong and R. T. Yang, ATChE 1. 33(6), 1045"(1987).
obtained.
18. M. M. Hassan, N. S. Raghavan, and D. M. Ruthven, Chem. Eng. Sci. 42(8), 2037 (1987).
The existence of multioie steady state has certain oracticai implications,
19. S. Farooq, M. M. Hassan, and D. M. Ruthven. Chem. Eng. Sci. 43(5). 1017 (1988).
since it means that uncter certain conditions the operation may be unstable to
a perturbatmn m feed composition or flow rate. Furthermore, if a unit is shut 20. H. S. Shin and K. 5. Knaebel, A/Ch£ J. 34(9), 1409 (1988).
down during ooerat1on, it may be necessary to purge the beds before the 21. A. Kapoor and R. T. Yang, Chem. Eng. Sci. 44(8), 1723 (1989).
opera·t,on can be successfully resumed with the desired oroctuct ourity. An
22. S. Farooq, D. M. Ruthven, and H. A. Boniface, Chem. Eng. Sci. 44(12), 2809 (1989).
23. J. L. L. Liow and C. N. Kenney, ATChE J. 36(1), 53 (1990).
Nole: A novei mathemalical anaylsis thal provides for the direct delerminat1on and stahilitv 24. M. W. Ackley and R. T. Yang, A/Ch£ J. 36(8), 1229 0990).
analysis of cvclic steady states has recently been developed: D. T. Croft and M. D. U:Van, Chem.
25. J. A. Ritter and R. T. Yang, Ind. Eng. Chem. Res. 30(5), 1023 (1991).
Eng. Sci. On press). See also Proceedings of Fourth Imernational Conference on Adsorption,
Kyo<o, Mav 1992. 26. S. Farooq and D. M. Ruthven, Chem. Eng. Set. 46(9), 2213 (1991).