Page 39 - Pressure Swing Adsorption
P. 39
'I
14 PRESSlJRE SWING ADSORPTION FUNDAMENTALS OF ADSORPTION 15
8
Oxidized so, 0.12 0.12
6
Gas carbon
~ Untreated CMS
"- ------ "c, .__ 0.08
0 -- 0.08
E .,- <I
E 4 Untreated so, "- ,
~ <I I {\ Decolorizing
014 /t I \ carbon
2 Oxidized I I \ 0,04
-------------- J \ \. r,
-✓
0<---'----"c---"c------cc:---==~ ' ' "-
O 5 10 15 20 25 0 I 0 ,
p, kPa 0.1 1.0 10 10 2 10' 0.1 1.0 10 10'
Pore radius, nm
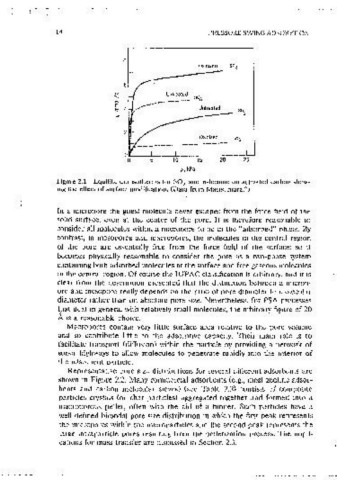
Figure 2.1 Equilibnum isotherms for SO and n-hexane on activated carbon show-
2
4
ing the effect of surface modificat1on. (Data from Mastsumura. ) (a) lb)
In a micropore the guest ffiotecule never escapes from the force field of the
.
solid surface, even at the center of the pore. lt is therefore reasonable to
consider all molecules within .a mtcrooore to be m the "adsorbed" ohase. By
contrast. in mesopores and macrooores, the molecules m the central region
of the pore are essentially free from the force field of the surface; so It "
<I
becomes physically reasonable to consider the pore as a two-ohase system "-
:,."
contammg both adsorbed molecules at the surface and free gaseous molecules <1
m the central region. Of course the lUPAC classificat1on 1s arbitrary, and ii 1s C
clear from the description oresenteJ that the distmctton between a micron~ 1'
ore and rnesooorc really deocn.ds on the ratio of pore diameter to moiccular \
diameter rather than on absoiute pore size. Nevertheless, for PSA orocesses
that deal m general with relatively small molecules, the arbitrary figure of 20 r \
A is a reasonable choice. 0
Macrooores contain very iittle surface area relative to the pore volume 10' 10' 10'
and so contribute little to the adsorptive capacity. Their mam role 1s to Pore radius (Al
facilitate transport (diffusion) within the oart1cie by providing a network of Jc)
super highways to allow molecules to penetrate rapidly mto the tntenor of
Figure 2.2 Pore size distributions for (a) typical activated carbons; (b) carbon
the adsorbent particle.
molecular sieve; (c) typical activated alumina.
Representative pore size distributions for several different adsorbents are
shown m Figure 2.2. Many commerciai adsorbents (e.g., most zeolitic adsor-
bents and carbon molecular sieves) (see Table 2.2) consists of composite
particles c1ystals (or char particles) aggregated together and formed into a
2. 1.4 Kinetically Selective Adsorbents
macroporous pellet, often with the aid of a binder. Such particles have a
well-defined bimodal pore size distribution m which the first peak represents While most adsorbents have a relatively wide distribution of pore size, kinetic
the m1cropores within the m1crooarticles and the second peak represents the selectivity deoends on stenc hindrance and therefore reomres a very narrow
large lntrapart1cle pores resulting from the pelletization process. The 1mpli- distribut10n of oore size. This 1s a charactenstic feature of zeolit1c adsorbents
cations for mass transfer are discussed in Section 2.3. since these matenals are crystalline and the dimensions of the rn1cropores are