Page 44 - Pressure Swing Adsorption
P. 44
18 PRESSURE SWING ADSORPTION FUNDAMENTALS OF ADSORPTION 19
The thermal acl1vat1on l)roccdurc is a two-step process m which volatile
matenal 1s first dnven off bv controlled pyrolysis followed by a controlled
"burnout" of the pores using oxidizing gases such as steam or CO at 800°C
2
(or even higher temperatures). 7 The surface of such activated carbons is
partially oxidized; so where a nonoolar surface 1s required, a further step 1s
often included, 10volving either evacuation or purging with an mert gas at •
elevated temperature. This eliminates most of the oxides as CO or CO •
2
In many liquid-phase applications activated carbon is used m powder ~onqmol i J
form, but for gas-phase applicatmns larger particles are needed. These are () Jj 40Q•C reqeneroted
made either directly bv crushing and screemng or more commonly by granu- ·--- 0 0 ertiylbenzene d
lation of the powder using binders such as pitch, which C<tn he act1valcd to () Cl ernyJbenu-ne .. uyre,.,e
$ ■ s1yrene =j
some extent during the finai thermai treatment. The orcparation of activated
carlxm m fiber form 1s a relat1vely new development which holds consider- ~
able nrom1se for the future. The diameter of the fibers 1s small ( ~ 10 µm) so i l
u ,o·
diffusional resistance is reduced to an mstgnificant level. To date such .
matenals do not appear to have been used m PSA orocesscs, but the raoid t•'---
kinetics make this an intngumg possibility. 0 •-
The nreparauon of carbon moiecuiar sieves (Figure 2.3) ts broadly similar '
but often mcludes an additional treatment with species such as benzene or "
•
'l'r■
10·' ,______________ •-:::::::_·
Coal
0 0 20 '0
Grinding Corb-On depos1t1on , mg- coroon / (J • MSC
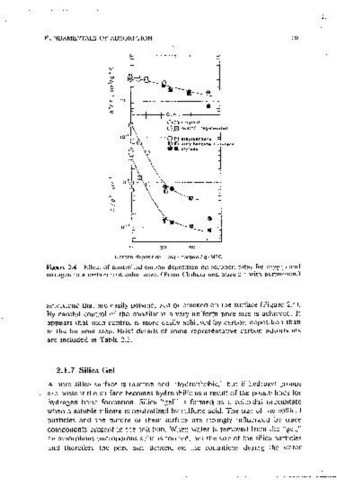
Figure 2.4 Effect of controlled carbon deposition on sorptJon rates for oxygen and
5
nitrogen in a carbon molecular sieve. (From Chihara and Suzuki, with oerm1ss1on.)
OJ.idal!on by Ai,
Ox1coal
Binder actelvlene that are easily oolymenzed or cracked on the surface (Figure 2.4).
0 D By careful controi of the conditions a very uniform pore size 1s achieved, It
Shapmg
appears that such control 1s more easily achieved :by carbon c1epos1tion than
0 m the burnout step. Brief details of some representative carbon adsorbents
Carbom:ullon are included in Tahle 2.3.
Urulo,m Initial Material
D 2.1. 7 Silica Gel
Sleam Acl1Yallon Treatment under
A pure silica surface 1s mactlve and "hyctroohobic," but if hydroxvl groups
0 Cracking Conditions are present the surface becomes hydrophilic as a result of the possibilities for
CMS H2 Q ol Hydrocarbons hydrogen bond format10n. Silica "gel" 1s formed as a colloidal precipitate
Accrvaled Carbon when a soluble silicate ,s neutralized bv sulfunc acid. The size of the co!lidal
CMS NZ particles and the nature of thetr surface are strongly mftuenced bv trace
CMS 02 components present rn the solutJOn. When water is removed from the "gel,"
Figure 2.3 Schematic diagram showmg the processes mvolved in the manufacture of an amorphous m1croporous solid ts formed, but the size of the silica oartides
carbon molecular sieve adsorbents. (From Junrgcn et ai.,1 with perm1ss1on.) and therefore the pore size depend on ttle conditions dunng ttle water