Page 49 - Pressure Swing Adsorption
P. 49
24
PRESSURE SWING ADSORPTION FUNDAMENTALS OF ADSORPTION 25
adsorption from the gas phase 1s an exothermic process; so eauilibrium favors
adsorption at lower temoeratUres and desorption at higher temperatures. At
sufficiently low concentratwn the equilibnum relattonshio generally ap~ l EB :1211-1
proaches a linear form (Henry's Law): -
ll = K'p or a = Kc (2.1) 0 1/0 1/0 1/0 i/0 1.0
PtPs
and the constant of oroport1onality ( K' or K) ts referred to as the "Henry·s
Figure 2.9 The Bntnauer classificauon of isotherms.
Law" constant or simply the Henry constant. It 1s evident that the Henry
constant is simply the adsorption eauilibnum constant, and the temperature
dependence can be expected to follow the usual vant Hoff relations:
2.2.2 Brunauer' s Classification
(2.2)
At higher concentrat10ns the equilibrium relationshio becomes curved.
where 11 H = 6.U - RT 1s the enthalpy change on adsorptmn. (For an Brunauer ciassifiect the commonly observed forms of 1sothenn mto the five
exothermic process !),.J-/ and /1U are negative, and the Henry constant types illustrated in Figure 2.9. Reference to the isotherm for water vapor
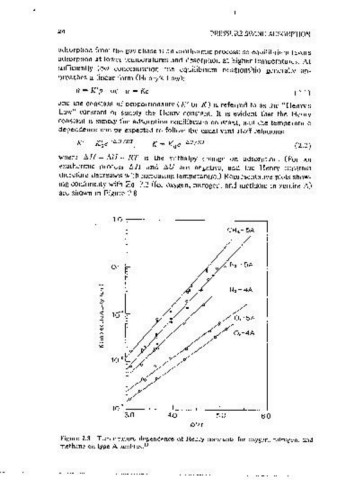
therefore decreases with mcreasmg temoerature.) Reoresentative plots show- (Figure 2.5) shows that H 0-4A 1s type I, H 0-a!lumma is type II, while
2
2
mg conformity with Ea. 2.2 (for oxygen, mtrogen, and methane m zeolite A) H 0~silica gel 1s type IV. Type I is charactenst1c of chem1sorot1on, where
2
are shown m Figure 2.8. the saturation limit corresponds to occupatmn of all surface s1tes, or to
physical adsorption in a microporous material wh~re the saturation limit
corresponds to complete filling of the m1cropores. Type Ill behavior corre-
,.o sponds to the• situation where the sorbate-surface mteractton 1s weaker then
CH 4 -5A the sorbate-sorbate mteract10n, as, for example, in the adsorotion of water
vaoor on a carbon surface. In a PSA system the isotherms are generally of
/ type l or type II form, and further discussion is therefore rcstnctcd to these
"' t 0 ,. Nz-5A cases.
2.2.3 ''Favorable" and "Unfavorable" Equilibria
j N'l.-4A In the analysis of adsorption coiumn dynamics it IS convement to classifv
"' . 0 • / adsorption equilibria as "favorable," "linear," or "unfavorable" depending
·~
~ on the shape of the dimensionless ( x-v) equilibrium diagram. The mean mg
•
s ,o"~ • 0.-5A of these terms 1s evident from Figure 2.10. (ln the "favorahle" case the
u
-" dimens10niess adsorbed phase concentrat1on 1s a!Wavs greater than the <li-
0 w 0,-4A
E menstonless fluid ohase concentration.) This classificat1on assumes that the
;;:: direction of mass transfer 1s from fluid phase to adsorbed Phase (i.e., an
adsorption process). Since for desorption the mitrnl .ind final states are
10-•
reversed, an isotherm that is "favorable" for actsorpt10n will be "unfavorable"
for desorot10n and vice versa.
2.2.4 Langmuir Isotherm
For m1croporous adsorbents the isotherm can often he rcprcscntccl, <ii least
approximately, by the ideal Langmuir modei:
'L ~
Figure 2.8 Temperature dependence of Henry constants for oxygen, nitrogen, and be (2.3)
q, 1 + be
methane on type A zeolites_ D