Page 45 - Pressure Swing Adsorption
P. 45
,Ii
20 PRESSURE SWING ADSORPTION FUNDAMENTALS OF ADSORPTION 21
Table 2.3. Physical Properties of Some Common Adsorbents
rcsultmg surface is no longer hydrophilic. Despite ns widespread use as u
Sp. pore Av. pore Pore Sp. Particle desiccant silica gel ,s not commoniy used in PSA orocesses as its physical
vol. diam. size area density strength 1s mfenor to that of alumma or zeolite based desiccants.
Adsnrhcnt (cm·; g- 1 ) (A> distrih. (m2 g-1) (gem • T\
'
Silica gel (I) 0.43 22 Ununodal 800 l.09 2. 1.8 Activated Alumina
Silica )?el (2) 1.15 140 Unimodal 340 0.62
2
An. alumw,i 0.50 JO-· ,ooo U111m11d11I :no 1.21-1 Activated alum ma 1s essentially a m1croporous (amorphous) form of A 1 0~
Acl. carh1m 0.15-0.5 Wide Bim11dal 200- 0.6~-0. 1 ) and 1s made hy several different methods. The -most common route 1s hy
range 2000 controlled dchvdrat1on of the trihvdrate A1 0:: 3H 0 formed m the Baver
2
2
CMS 0.25 Dimodal 400 0.98 process but some alummas are made by orec101tat1on from a soluble salt in a
manner similar to the production of silica gel.
2.1.9 Zeolites
removal step. Bnef details of two representative matenals are mciuded 111
Table 2.3. The large-pore matenal is used in many liquid-phase applications. In contrast to the other adsorbents so far considered, the zeolites are
whiie the small-pore matenal ts widely used as a desiccant in vaoor-ohase crystalline rather than amorphous. and the m1cro@ores are actuallv mtracrvs-
systems. talline channels with dimr:ns1ons precisely deten:nmcd by the crvstai struc-
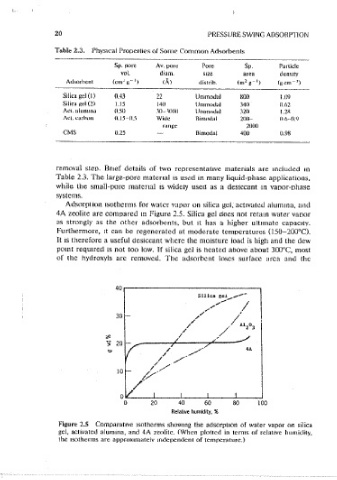
Adsorption isotherms for waicr vapor on silica gci, acuvaicd alumina, and ture. There is therefore virtually no disirihution of microporc size, and these
4A zeolite are compared m Figure 2.5. Silica gci docs not retam water vapor adsorbents show well~dcfined s1ze~selcct1ve mo·lecular sieve properties-
as strongly as the other adsorbents, but Jl has a higher ultimate capacity. exclusion of molecules larger than a certam cnttcal size and strong stenc
Furthermore, 1t can be regenerated at moderate temoeratures (150-200°C). restnct1on of diffusion for molecules with dimensions approaching this limit.
It JS therefore a useful desiccant where the moisture load is high and the dew The framework structures of three of the most important zeolites are shown
pomt required is not too low. If silica gei is heated above about 300°C, most schematically m Figure 2.6. The frameworks consist of tetrahedrally con-
of the hydroxyls arc removed. The adsorhcnt loses surface area and the nected assemblages of Si0 1 and A\0 1 units. To translate the schematic
diagrams into aciual structur~s one musi-consider that the iines represent the
diameters of oxygen atoms (or ions), while the m:uch smaller Si or Al atoms
40~--------------~ are located at the ap1ces of the ooiyhedra. Within rather broad iimits Si and
Al atoms are interchangeable in the lattice, hut each Al introduces u net
negative charge that must be balanced tw an exchangeable catwn. In many
30 structures, notably zeolite A, the exchangeable cations oart1ally (or totally)
obstruct the mrcrooores. The eouilibnum distribution of the exchangeable
catmns among the various possible cat10n "sites" has been extensively stud-
9
ied and is well established for most of the common zeolites. For example, 10
4A zeolite A there are three types of site, as indicated in Figure 2.6(a). The most
2
favorable are the type I sites (eight per cage) so in the Ca + form (s,x cations
per cage) all cat10ns can be accommodated. m the type I sites where thev do
not obstruct the channels. The effective ditnens:1on of the channei 1s then
limited by the aperture of the eight-membered oxygen nng (wmdow), which
has a free diameter of about 4.3 A. Since moiecules with diameters up to
80 about 5.0 A can penetrate these windows, this 1s referred to as a "5A" sieve.
Relative humidity,% The Na+ form contains 12 cat10ns per cage; so not only are all eight type I
Figure 2.5 Comparative isotherms showmg the adsorpuon of water vapor on silica sites filled, but all wmdow sites (3.0 per cage) are also filled. (The twelfth
gei, aclivated a\umma, and 4A zcolitc. (When plotted in terms of rciat1vc humidity, Na+ catmn 1s accommodated in the relat1vcly unfavorable type III site.) The
the isotherms are approx1mateiv independent of temperature.) Na+ cation partially obstructs the wmdows, reducmg the effective size cutoff