Page 41 - Pressure Swing Adsorption
P. 41
16 PRloSSLIRE SWING ADSORPTION FUNDAMENTALS OF ADSORPTION 17
distribution of pore size. This unifornutv of pore size 1s achieved in two wavs:
(d)
by careful control of the conditions ctunng the act1vat1on step and by
.OlS controlled ctepos1t1on of easily crackable or oolvmenzable hvctrocarbons such
as acetylene. Control of these processes provides the means by which the
pore size can be adjusted.s.ei In this resoect there 1s somewhat greater
flexibility than with crystalline m1crooorous materiais rn which the oore
dimensions are fixed bY the ccystai structure. ln kinetically selective adsor-
Por<> Vol •• 0\0
bents the onmary parameters deterrnmmg the sel'ect1v1tv are the pore size
\ secondary importance. Thus, desmte the difference m chem1ca1 nature,
and pore size distribution. The nature of the :matenal 1s generally of
. oos small-oore zeolites and molecular sieve carbons exhibit very s11Tlilar kinetic
\ selectivities.
I
\
~ 2.1.5 Phystcal Strength
..
O. 000 __.__........,...Lu .;..o.L....__._ .u.J ___ ,_,___._J...,.......,~, ....
,001 .Ol Reoeated pressurization and deoressunzat1on of an adsorbent bed tends to
Poro Olom.ilor '()' (M!crona) cause attrition of the adsorbent particles. Physical strength 1s therefore a
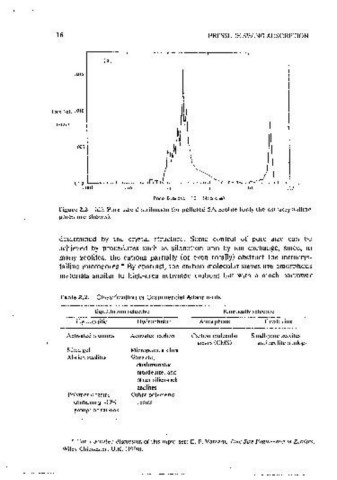
Figure 2.2 (d). Pore size distrihut1on for pelleted SA i.colitc (only the cxtracrystallinc pnme consideration m the ct101cc of an adsorhcnt for a PSA procc,;s. Such
pores arc shown). considerat10ns may mdeed preclude the use of an otherwise des,rablc adsor•
bent m favor of a matenal that, from kinetic and eauilibnum considerations
aione. may appear to have mferior properties. Both the "crush strength" and
determined by the crystal structure. Some control of pore size can be
the "abrasion resistance" are strongly deoenctent on the way in which the
achieved by procedures such as silanat1on and by 10n exchange, smce, m
adsorbent oart1cles are manufactured, mcluding such factors as the nature of
many zeolites, the cations partially (or even totallv) obstruct the mtracrys-
the binder and the pretreatment conditions, but only very limited infonnatton
talline microoores."' By contrast, the carbon molecular sieves are amorphous
1s available in the open literature.*
matenals similar to high-area activated carbons but with a much narrower
2.1.6 Activated Carbon and Carbon Molecular Sieves
Table 2.2. Classification of Comrnerc1al Adsorbents
Activated carbon is produced m many different forms that differ mamiy m
Equilibrmm selective Kinet1cally selective
pore size distribution and surface polarity. The nature of the final product
Hydrophilic Hydrophobic Amorphous Crv.~talline
depends on both the starting material and the aci1vat1on proccclurc. For
Activated alumina Activated carbon Carbon molecular Small-pore zeoli1es liquid-phase adsorption a relat1vely large oore size ts required, and such
SICVCS WMS) anti 7.Colitc anaings matenals can be made by both thermal and chemical activation procedures
Silica gel Mkroporous silica from a wide range of carbonaceous starting materials. The activated carhons
Al-rich zeolites Silica\ite, used in gas adsorption generally have much smaller pores, with a substantial
dealum1nated
mordeoite, and fraction of the total ooros1tv m the m1crooore range. These adsorbents are
other silica-rich generally made by thermal activation from a relatively dense form of carbon
zeolites such as bitummous coal. High·area small~pore carbons mav also tie made
Polymenc resins Other polvmenc from sources such as coconut shells, but the product generally has insufficient
comammg-OH resins
physical strength for PSA applicallons.
groups or cations
• A useful reference 1s: C. W. Roberts, "Moiecuiar Sieves for lndustrral App!icaiions." In
~ For :i deiailed discussmn of this topic:, see: E. F. Vansan(. Pore Size EnKmeerm,: m Zeolites, Properties and Applicatum.1· of Zeo/ites, R. P. Townsend, ed., Sp·ecial Puhl. No. 33, The Chemical
Wiley Chichester, U.K. (1990). Society, London (198/IJ.