Page 48 - Pressure Swing Adsorption
P. 48
22 PRESSURE SWING ADSORPTION FUNDAMENTALS OF ADSORPTION 23
'<'
e: 0,20
"'
u
u
~ 0.15
.E -p-xylene
"' -benzene
.S
.,, 0.10
~
!
- 2,3 dimethyl butene
i5 0.05
n
- cyc!ohexone
Jl
o, w,_,_ _ _,_ _ _,__ID: o-xv.iene
5 6 7
(al lb) ' Kinetic Diameter rAl
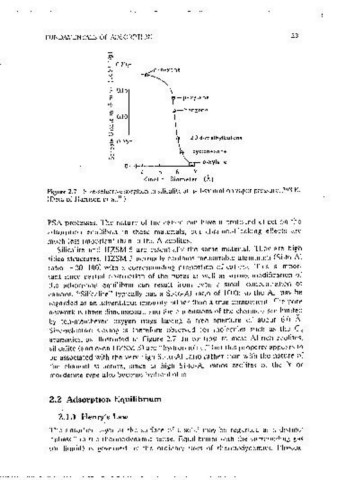
Figure 2.7 Size-selective sorpt1on in silicalitc at half-saturation vapor pressure, 298 K.
(Data of Harnson ei ai. 10 )
PSA processes. The nature of the cation can have a profound effect on the
adsorotton equilibria 10 these matenals, but channel-blocking effects are
much less important than 1n the A zeolites.
Silicalite ~nd HZSM-5 are essentially the same material. Thev are high
silica structures. HZSM-5 normally contains measurable alumrnum (Si-to-Al
ratio ~30-100) with a corresooncling nrooort1on of cations. This 1s 1moor-
tant since partial obstruction of the pores as well as strong modificanon of
the adsorotJon equilibna can result from even a small concentration of
cations. "Silicalite" typ1cally has a Si-to-Al ratio :of 1000; so the AI may be
regarded as an adventltous ,mounty rather than a· true comoonenL The pore
lei network is three dimensional, and the dimensions: of the channels are lim1t~d
Figure 2.6 Schematic diagrams showmg the framework structures of three common by ten-membered oxygen nngs having a free ·aperture of about 6.0 A.
zeoiites. (a) Zeolite A (the three exchangeable cation sites are mdicated), (b) Zeolite Size-selective sieving 1s therefore observed for .molecules such as the Cfl
X or Y. (c) silicalitc or ZSM-5. More detailed descnpilons of these structures are
given by Brcckll as well as m more recent reviews. aromatics. as illustrated m Figure 2.7. Jn contrast to most Al-nch zeolites.
silicalite (and even HZSM-5) arc ''hydrophobic," hut this pronertv appears to
be associated with the very high Si-to-Al ratio rather than with the nature of
the channel structure, smce at high Si-to-Al ratios zeoiites of the Y or
to about 4 A-hence the term 4A sieve. Reolacement of Na+ bv the iarger
morctenite type also become hydrophobic.
K+ catmn reduces the dimensions even further so that only water an(! other
very small molecules such as NH can penetrate at an appreciable rate (3A).
1
The framework structures of X and Y zeolites are the same, and these
2.2 Adsorption Equilibrmm
materiais differ only in the Si-to-Al ratio-and therefore m the number of
exchangeable cat10ns. The pore structure is very ooen, the ~onstructions 2.1.1 Henry's Law
bemg twelve-membered oxygen rmgs with free diameter ~ 7.5 A. Molecules
with diameters up to abom 8.5 A can penetrate these channeis with little The adsorbed layer at the surface of a solid may be regarded as a distinct
steric hindrance, and this inciudes all common gaseous species. Size-selective "phase" m the thermodynamic sense. Eouilihnum with the surrounding gas
sieving 1s observed for larger molecules, but such effects are not relevant to (or liquid) 1s governed tiv the ordinary laws of thermodvnam,cs. Phvs,cai