Page 136 - Principles of Applied Reservoir Simulation 2E
P. 136
Part II: Reservoir Simulation 121
a saturated hydrocarbon, that is, it has a single bond between carbon atoms.
Examples include methane and ethane. Paraffins have the general chemical
formula C nH 2n+2. Napthenes are saturated hydrocarbons with a ringed structure,
as in cyclopentane. They have the general chemical formula C nH 2n. Aromatics
are unsaturated hydrocarbons with a ringed structure that have multiple bonds
between the carbon atoms as in benzene. The unique ring structure makes aro-
matics relatively stable and unreactive.
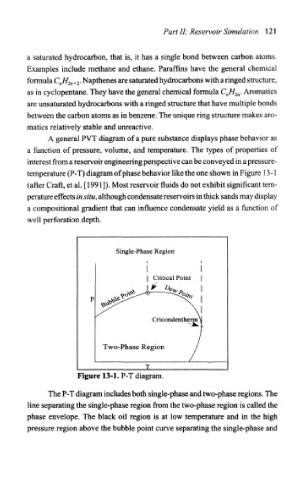
A general PVT diagram of a pure substance displays phase behavior as
a function of pressure, volume, and temperature. The types of properties of
interest from a reservoir engineering perspective can be conveyed in a pressure-
temperature (P-T) diagram of phase behavior like the one shown in Figure 13-1
(after Craft, et al. [1991]). Most reservoir fluids do not exhibit significant tem-
perature effects in situ, although condensate reservoirs in thick sands may display
a compositional gradient that can influence condensate yield as a function of
well perforation depth.
Single-Phase Region
i
I
| Critical Point
Two-Phase Region
Figure 13-1. P-T diagram.
The P-T diagram includes both single-phase and two-phase regions. The
line separating the single-phase region from the two-phase region is called the
phase envelope. The black oil region is at low temperature and in the high
pressure region above the bubble point curve separating the single-phase and