Page 213 - Principles of Catalyst Development
P. 213
202 CHAPTER 8
where there are two types of sites, with the most active eliminated first. An
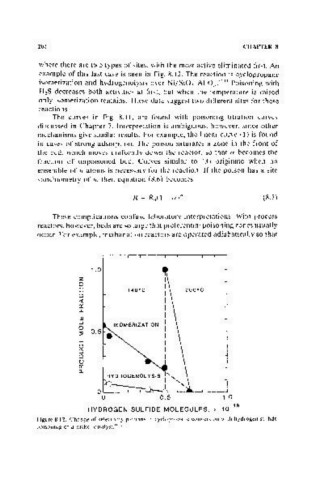
example of this last case is seen in Fig. 8.12. The reaction is cyclopropane
isomerization and hydrogenolysis over Ni/SiO r AI 20 J • (611 Poisoning with
H 2S decreases both activities at flrst, but when the temperature is raised
only isomerization remains. These data suggest two different sites for these
reactions.
The curves in Fig. 8.11, are found with poisoning titration curves
discussed in Chapter 7. Interpretation is ambiguous, however, since other
mechanisms give similar results. For example, the linear curve (1) is found
in cases of strong adsorption. The poison saturates a zone in the front of
the bed, which moves uniformly down the reactor, so that a becomes the
fraction of unpoisoned bed. Curves similar to (3) originate when an
ensemble of 11 atoms is necessary for the reaction. If the poison has a site
stoichiometry of 11, then equation (8.6) becomes
R = Roll - a)" (8.7)
These complications confuse laboratory interpretations. With process
reactors, however, beds are so large that preferential poisoning zones usually
occur. For example, methanation reactors are operated adiabatically so that
1.0
z
0
I-
()
«
cr
u..
W
...J
0
~ 0.5
I-
()
:::)
0
0
a:
a..
1.0
HYDROGEN SULFIDE MOLECULES, x 10- 19
Figure 8.12. Change or selectivity patterns in cyclopropane conversion with hydrogen sulfide
poisoning or a nickel catalyst. ((.11