Page 101 - Principles of Catalyst Development
P. 101
88 CHAPTER 5
selectivity. Searching further, the analysis shows a possible alternative
reaction
(5.2)
involving only oxygen insertion and dehydrogenation. This may proceed
directly or involve a combination of the next reaction, with CH]OH as an
intermediate that decomposes via the last reaction. At this point, one
possibility is to focus upon equation (5.2) as the target.
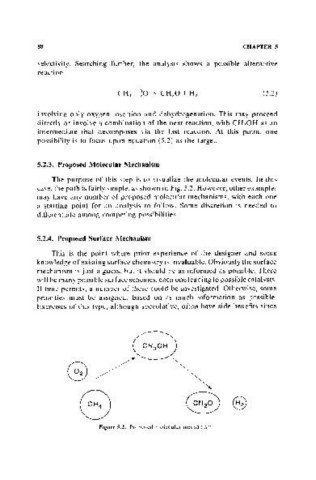
5.2.3. Proposed Molecular Mechanism
The purpose of this step is to visualize the molecular events. In this
case, the path is fairly simple, as shown in Fig. 5.2. However, other examples
may have any number of proposed molecular mechanisms, with each one
a starting point for an analysis to follow. Some discretion is needed to
differentiate among competing possibilities.
5.2.4. Proposed Surface Mechanism
This is the point where prior experience of the designer and some
knowledge of existing surface chemistry is invaluable. Obviously the surface
mechanism is just a guess, but it should be as informed as possible. There
will be many possible surface schemes, each one leading to possible catalysts.
If time permits, a number of these could be investigated. Otherwise, some
priorities must be assigned, based on as much information as possible.
Exercises of this type, although speculative, often have side benefits since
--- ....
/'
( '" " \
I CH 0H ,
3
\ I
G //'----/~
8 9@
Figure 5.2. Proposed molecular mechanism.