Page 102 - Principles of Catalyst Development
P. 102
DESIGN OF CATALYSTS 89
gaps in existing data become apparent and lead to ideas for future research.
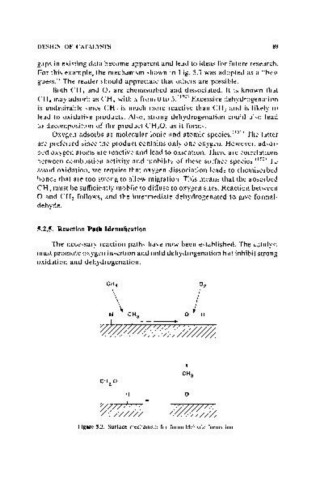
For this example, the mechanism shown in Fig. 5.3 was adopted as a "best
guess." The reader should appreciate that others are possible.
Both CH 4 and O 2 are chemisorbed and dissociated. It is known that
CH 4 may adsorb as CH, with x from 0 to 3.(150) Excessive dehydrogenation
is undesirable since CH 2 is much more reactive than CH 3 and is likely to
lead to oxidative products. Also, strong dehydrogenation could also lead
to decomposition of the product CH 20, as it forms.
Oxygen adsorbs as molecular ionic and atomic speciesYSl) The latter
are preferred since the product contains only one oxygen. However, adsor-
bed oxygen atoms are reactive and lead to oxidation. There are correlations
between combustion activity and mobility of these surface speciesY 52 ) To
avoid oxidation, we require that oxygen dissociation leads to chemisorbed
bonds that are too strong to allow migration. This means that the adsorbed
CH 3 must be sufficiently mobile to diffuse to oxygen sites. Reaction between
o and CH 3 fo\1ows, and the intermediate dehydrogenated to give formal-
dehyde.
5.2.5. Reaction Path Identification
The necessary reaction paths have now been established. The catalyst
must promote oxygen insertion and mild dehydrogenation but inhibit strong
oxidation and dehydrogenation.
j
o
H
?@/w- ~
Figure S.3. Surface mechanism for formaldehyde formation.