Page 117 - Principles of Catalyst Development
P. 117
CATALYST PREPARATION 105
phase changes occur, simultaneously with loss of hydroxyl groups. This
results in 1)-, )1-, and 8-phases approximately at the temperatures shown.
Exact transition points depend on many preparational factors and are
difficult to define. These oxides are known as the ")I-group" or "Iow-
temperature" aluminas. Structures are very similar, with a formula
AI 10]nH 20, in which n = 0.6-0. All are based on cubic close packing of
oxygen ions (ABCABC) but are best described as a defect spinel,
AI[Alsn£ )1/3)0 4 , where AI occupies tetrahedral or octahedral interstices
and [ ] is a cation vacancy. Different phases are distinguished by variations
in relative intensities of key diffraction lines.
Above 1000°C, monoclinic 8-AI 20] forms, transforming to hexagonal
(ABABAB) a-A1 20 3 at 1200°C. These are anhydrous, low surface area
oxides and are not suitable for porous supports. They are used in applica-
tions where mechanical strength, and not high surface area, is required.
The acidity of these aluminas was discussed in Chapter 4. In particular,
the effect of calcination temperature on acid strength distribution is shown
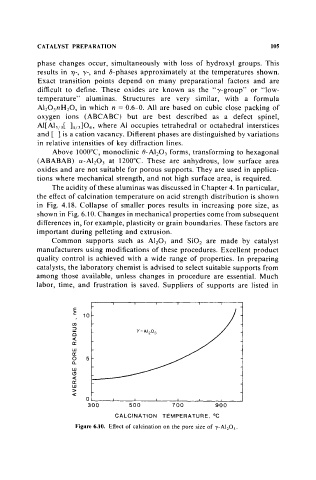
in Fig. 4.18. Collapse of smaller pores results in increasing pore size, as
shown in Fig. 6.10. Changes in mechanical properties come from subsequent
differences in, for example, plasticity or grain boundaries. These factors are
important during pelleting and extrusion.
Common supports such as AI 20 J and Si0 2 are made by catalyst
manufacturers using modifications of these procedures. Excellent product
quality control is achieved with a wide range of properties. In preparing
catalysts, the laboratory chemist is advised to select suitable supports from
among those available, unless changes in procedure are essential. Much
labor, time, and frustration is saved. Suppliers of supports are listed in
E
c 10
(/)
:::J
D
«
cr:
UJ
cr:
0 5
Q.
UJ
C)
«
cr:
UJ
>
«
0
300 500 700 900
CALCINATION TEMPERATURE, °C
Figure 6.10. Effect of calcination on the pore size of ,},-A1 20 3 •