Page 119 - Principles of Catalyst Development
P. 119
CATALYST PREPARATION 107
Both nickel and aluminum preCIpitate hydrous oxide sols. For best
combinations, care must be taken to ensure that precipitation occurs simul-
taneously. Variations of pH within the vessel or during addition of the alkali
result in preferential precipitation of one component, yielding product
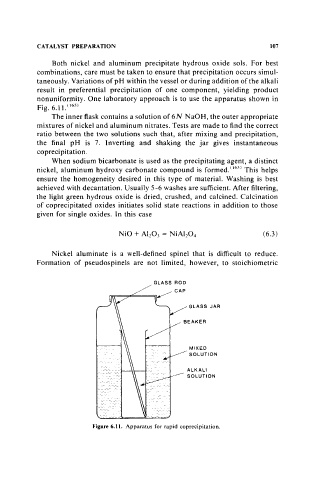
nonuniformity. One laboratory approach is to use the apparatus shown in
Fig. 6.11.(165)
The inner flask contains a solution of6N NaOH, the outer appropriate
mixtures of nickel and aluminum nitrates. Tests are made to find the correct
ratio between the two solutions such that, after mixing and precipitation,
the final pH is 7. Inverting and shaking the jar gives instantaneous
coprecipitation.
When sodium bicarbonate is used as the precipitating agent, a distinct
nickel, aluminum hydroxy carbonate compound is formed. 1163 ) This helps
ensure the homogeneity desired in this type of material. Washing is best
achieved with decantation. Usually 5-6 washes are sufficient. After filtering,
the light green hydrous oxide is dried, crushed, and calcined. Calcination
of coprecipitated oxides initiates solid state reactions in addition to those
given for single oxides. In this case
(6.3)
Nickel aluminate is a well-defined spinel that is difficult to reduce.
Formation of pseudospinels are not limited, however, to stoichiometric
GLASS ROD
GLASS JAR
MIXED
SOLUTION
ALKALI
SOLUTION
Figure 6.11. Apparatus for rapid coprecipitation.