Page 122 - Principles of Catalyst Development
P. 122
110 CHAPTER 6
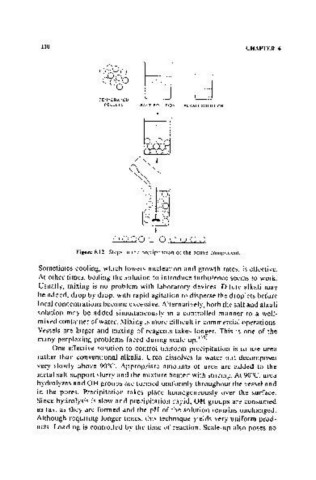
DEHYDRATED
PELLETS SALT SOLUTION ALKALI SOLUTION
~
l@f
~
l~JfJ
,
0000000000
Figure 6.12. Steps in the precipitation of the active component.
Sometimes cooling, which lowers nucleation and growth rates, is effective.
At other times, boiling the solution to introduce turbulence seems to work.
Usually, mixing is no problem with laboratory devices. Dilute alkali may
be added, drop by drop, with rapid agitation to disperse the droplets before
local concentrations become excessive. Alternatively, both the salt and alkali
solution may be added simultaneously in a controlled manner to a well-
mixed container of water. Mixing is more difficult in commercial operations.
Vessels are larger and mixing of reagents takes longer. This is one of the
many perplexing problems faced during scale-up. (35)
One effective solution to control uniform precipitation is to use urea
rather than conventional alkalis. Urea dissolves in water but decomposes
very slowly above 90°C. Appropriate amounts of urea are added to the
metal salt-support slurry and the mixture heated with stirring. At 90°C, urea
hydrolyzes and OH groups are formed uniformly throughout the vessel and
in the pores. Precipitation takes place homogeneously over the surface.
Since hydrolysis is slow and precipitation rapid, OH groups are consumed
as fast as they are formed and the pH of the solution remains unchanged.
Although requiring longer times, this technique yields very uniform prod-
ucts. Loading is controlled by the time of reaction. Scale-up also poses no