Page 127 - Principles of Catalyst Development
P. 127
CATALYST PREPARATION 115
?fl.
Z
0
(J)
a:
w 50
>
z
0
<..>
w
z
w
::i:
::> 0
<..> 0 10 20 30
PERCENT H POSITIONS
EXCHANGED WITH LA
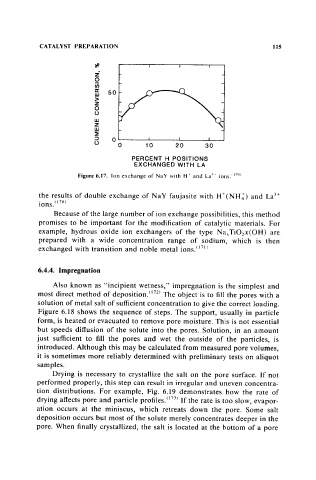
Figure 6.17. Ion exchange of NaY with H+ and La)' ions." 70 )
the results of double exchange of NaY faujasite with H+(NH1) and La3+
ions. 1170 )
Because of the large number of ion exchange possibilities, this method
promises to be important for the modification of catalytic materials. For
example, hydrous oxide ion exchangers of the type Na xTi0 2x(OH) are
prepared with a wide concentration range of sodium, which is then
exchanged with transition and noble metal ions. (171)
6.4.4. Impregnation
Also known as "incipient wetness," impregnation is the simplest and
most direct method of deposition.(l72) The object is to fill the pores with a
solution of metal salt of sufficient concentration to give the correct loading.
Figure 6.18 shows the sequence of steps. The support, usually in particle
form, is heated or evacuated to remove pore moisture. Thiis is not essential
but speeds diffusion of the solute into the pores. Solution, in an amount
just sufficient to fill the pores and wet the outside of the particles, is
introduced. Although this may be calculated from measured pore volumes,
it is sometimes more reliably determined with preliminary tests on aliquot
samples.
Drying is necessary to crystallize the salt on the pore surface. If not
performed properly, this step can result in irregular and uneven concentra-
tion distributions. For example, Fig. 6.19 demonstrates how the rate of
drying affects pore and particle profilesY731 If the rate is too slow, evapor-
ation occurs at the miniscus, which retreats down the pore. Some salt
deposition occurs but most of the solute merely concentrates deeper in the
pore. When finally crystallized, the salt is located at the bottom of a pore