Page 130 - Principles of Catalyst Development
P. 130
1t8 CHAPTER 6
0 100r----.-----r-----r----.-----~~
UJ
U
::J
0
UJ
a::
....J 50% NIOI AI 20 3
UJ REDUCTION AT 400·C
~
S?
Z 50
lL.
0
UJ
Cl
<I:
~
Z
UJ
u
a::
UJ
Q.
OL---~----L-~~~~~--~~
300 400 700
CALCINATION TEMPERATURE. °C
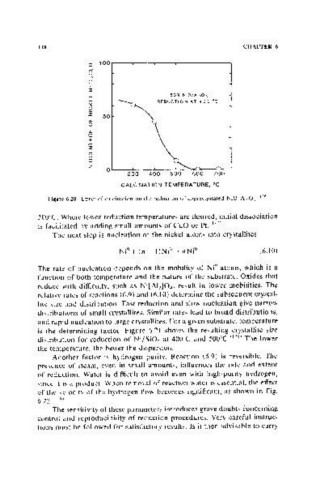
Figure 6.20. EHeet of calcination on the reduction of coprecipitated NiO-AI,O). i 176)
300°C. Where lower reduction temperatures are desired, initial dissociation
is facilitated by adding small amounts of CuO or Pt. (1771
The next step is nucleation of the nickel atoms into crystallites
(6.10)
The rate of nucleation depends on the mobility of Nio atoms, which is a
function of both temperature and the nature of the substrate. Oxides that
reduce with difficulty, such as Ni[AI 2]04, result in lower mobilities. The
relative rates of reactions (6.9) and (6.10) determine the subsequent crystal-
lite size and distribution. Fast reduction and slow nucleation give narrow
distributions of small crystallites. Similar rates lead to broad distributions,
and rapid nucleation to large crystallites. For a given substrate, temperature
is the determining factor. Figure 6.21 shows the resulting crystallite size
distribution for reduction of Ni/Si0 2 at 400°C and 500°c.(1781 The lower
the temperature, the better the dispersion.
Another factor is hydrogen purity. Reaction (6.9) is reversible. The
presence of steam, even in small amounts, influences the rate and extent
of reduction. Water is difficult to avoid even with high-purity hydrogen,
since it is a product. When removal of reaction water is essential, the effect
of the velocity of the hydrogen flow becomes significant, as shown in Fig.
6.22.117HI
The sensitivity of these parameters introduces grave doubts concerning
control and reproducibility of reduction procedures. Very careful instruc-
tions must be followed for satisfactory results. Is it then advisable to carry