Page 133 - Principles of Catalyst Development
P. 133
CATALYST PREPARATION 121
of the material. Examples are high-loading dispersed metals, porous metals,
and reduced fused oxides.
6.5.1. High Loading Dispersed Metals
Coprecipitated oxide systems such as NiO-AI 20 3 may be prepared
over the complete composition range. High concentration, highly dispersed
metal catalysts are produced by reducing the oxide structure. Compositions
as high as 70-80 wt % Nil AI 20 3 with crystallite sizes of 2-3 nm are formed
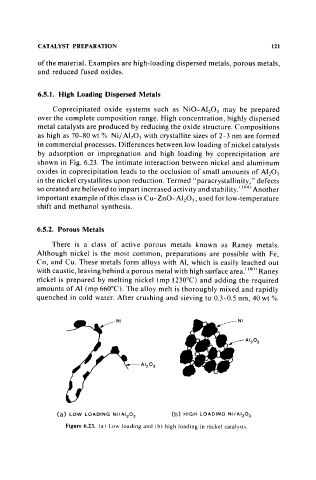
in commercial processes. Differences between low loading of nickel catalysts
by adsorption or impregnation and high loading by coprecipitation are
shown in Fig. 6.23. The intimate interaction between nickel and aluminum
oxides in coprecipitation leads to the occlusion of small amounts of Al 20 3
in the nickel crystallites upon reduction. Termed "'paracrystallinity," defects
so created are believed to impart increased activity and stability. (164) Another
important example of this class is Cu-ZnO-AI 20 3 , used for low-temperature
shift and methanol synthesis.
6.5.2. Porous Metals
There is a class of active porous metals known as Raney metals.
Although nickel is the most common, preparations are possible with Fe,
Co, and Cu. These metals form alloys with AI, which is easily leached out
with caustic, leaving behind a porous metal with high surface area. (180) Raney
n'ickel is prepared by melting nickel (mp 1230°C) and adding the required
amounts of Al (mp 660°C). The alloy melt is thoroughly mixed and rapidly
quenched in cold water. After crushing and sieving to 0.3-0.5 nm, 40 wt %
(a) LOW LOADING Ni/AI 2 0 3 (b) HIGH LOADING Ni/AI 2 0 3
Figure 6.23. (a) Low loading and (b) high loading in nickel catalysts.