Page 17 - Principles of Catalyst Development
P. 17
CATALYTIC FUNCTIONS 3
100~--~----~----~----~----~----~--~
3H 2 +N 2 "2NH 3
z H 2 /N 2 =3
o 80
(f)
a:
w
> 60
z
o
()
~ 40
:)
a:
III
....J 20
:)
o
w
100 200 300 400 500 600 700
TEMPERATURE,OC
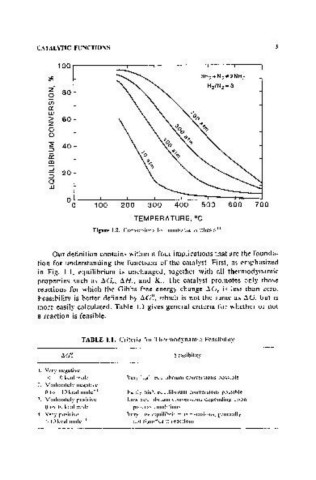
Figure 1.2. Conversions for ammonia synthesis(l)
Our definition contains within it four implications that are the founda-
tion for understanding the functions of the catalyst. First, as emphasized
in Fig. 1.1, equilibrium is unchanged, together with all thermodynamic
properties such as !l 0" !l H" and K r • The catalyst promotes only those
reactions for which the Gibbs free energy change !lOr is less than zero.
Feasibility is better defined by !lO~, which is not the same as !lOr but is
more easily calculated. Table 1.1 gives general criteria for whether or not
a reaction is feasible.
TABLE 1.1. Criteria for Thermodynamic Feasibility
Feasibility
1. Very negative
< - 10 kcal mole~' Very high equilibrium conversions possible
2. Moderately negative
o to -10 kcal mole-I Fairly high equilibrium conversions possible
3. Moderately positive Low equilibrium conversions depending upon
o to 10 kcal mole I process conditions
4. Very positive Ver) low equilibrium conversions, generally
> 10 kcal mole-- ' not signiiicant reactions