Page 27 - Principles of Catalyst Development
P. 27
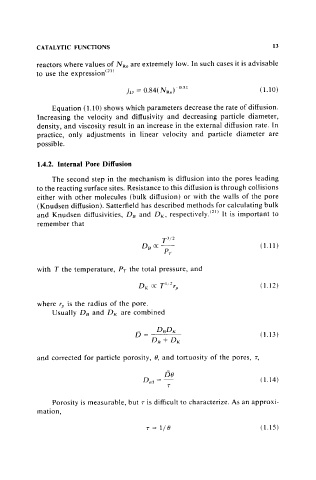
CATALYTIC FUNCTIONS 13
reactors where values of N Re are extremely low. In such cases it is advisable
to use the expression(21)
(LlO)
Equation (1.10) shows which parameters decrease the rate of diffusion.
Increasing the velocity and diffusivity and decreasing particle diameter,
density, and viscosity result in an increase in the external diffusion rate. In
practice, only adjustments in linear velocity and particle diameter are
possible.
1.4.2. Internal Pore Diffusion
The second step in the mechanism is diffusion into the pores leading
to the reacting surface sites. Resistance to this diffusion is through collisions
either with other molecules (bulk diffusion) or with the walls of the pore
(Knudsen diffusion). Satterfield has described methods for calculating bulk
and Knudsen diffusivities, D[j and D K , respectively.(21) It is important to
remember that
(1.11)
with T the temperature, PT the total pressure, and
(l.l2)
where r" is the radius of the pore.
Usually DB and DK are combined
(1.13)
and corrected for particle porosity, e, and tortuosity of the pores, T,
De
Dell =- ( 1.14)
T
Porosity is measurable, but T is difficult to characterize. As an approxi-
mation,
T = 1/ e (1.15)