Page 28 - Principles of Catalyst Development
P. 28
14 CHAPTER 1
is useful. If e is not known, sufficient accu'racy is achieved by the approxima-
tion e = 0.5. Thus
Dell = (0.25)0 (l.16)
is good enough!
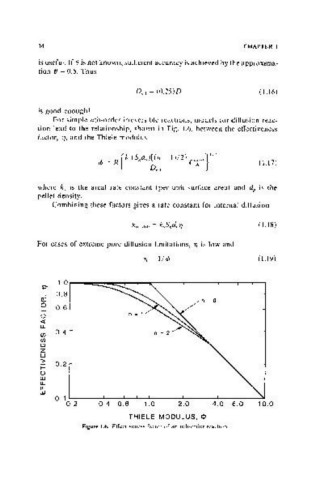
For simple nth-order irreversible reactions, models for diffusion-reac-
tion lead to the relationship, shown in Fig. 1.6, between the effectiveness
factor, 1], and the Thiele modulus
(1.17)
where k, is the areal rate constant (per unit surface area) and d p is the
pellet density,
Combining these factors gives a rate constant for internal diffusion
(1.18)
For cases of extreme pore diffusion limitations, 1] is low and
1]=I/c/> 0.19)
1.0~---=~~~--~~-----'------.----r~r-r
- 0,8
a:
o 0,6
f-
()
<{
lJ..
0.4
(j)
(j)
LJ.J
Z
LJ.J
> 0,2
f-
()
LJ.J
lJ..
lJ..
LJ.J 0.1 ~ ____ ~ ____ ~~ __ ~ ____ ~ ______ ~ __ ~ __ -L~
0.2 0.4 0,6 1.0 2.0 4.0 6,0 10.0
THIELE MODULUS, <l>
Figure 1.6. Effectiveness factor of an nth-order reaction,