Page 34 - Principles of Catalyst Development
P. 34
20 CHAPTER I
PELLET RATE, moles 9- 1 hr- I
10 4 ..
'Q on 'Q . '" 'Q
'0
10 3
2 Chemica I
10
Rate
Controlled
10
e
P[)2
T p
-2
10
-6 -~ -4 -3 -2 -I 4 ~
10 10 10 10 10 10 10 10 10
SURFACE RATE, moles 9- 1 hr- I
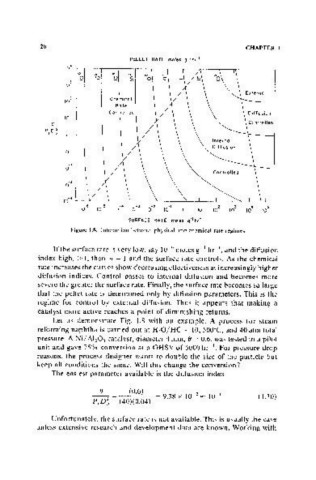
Figure 1.8. Interaction between physical and chemical rate regimes.
If the surface rate is very low, say 10- moles g -I hr--\ and the diffusion
6
index high, > 1, then TJ = 1 and the surface rate controls. As the chemical
rate increases the curves show decreasing effectiveness at increasingly higher
diffusion indices. Control passes to internal diffusion and becomes more
severe the greater the surface rate. Finally, the surface rate becomes so large
that the pellet rate is determined only by diffusion parameters. This is the
regime for control by external diffusion. Thus it appears that making a
catalyst more active reaches a point of diminishing returns.
Let us demonstrate Fig. 1.8 with an example. A process for steam
reforming naphtha is carried out at H 20/HC = 10, 500°C, and 40 atm total
pressure. A Nil Al 20 3 catalyst, diameter 4 mm, (J = 0.6, was tested in a pilot
unit and gave 75% conversion at a GHSV of 5000 hr-I. For pressure drop
reasons, the process designer wants to double the size of the particle but
keep all conditions the same. Will this change the conversion?
The easiest parameter available is the diffusion index
_(J_ = (0.6) = 9.38 x 10-2 = 10- 1 ( 1.30)
PTD~ (40)(0.04f
Unfortunately, the surface rate is not available. This is usually the case
unless extensive research and development data are known. Working with