Page 93 - Principles of Catalyst Development
P. 93
80 CHAPTER 4
REACTANT
PRODUCT
SELECTIVITY
RESTRICTED TRANSITION
STATE SELECTIVITY
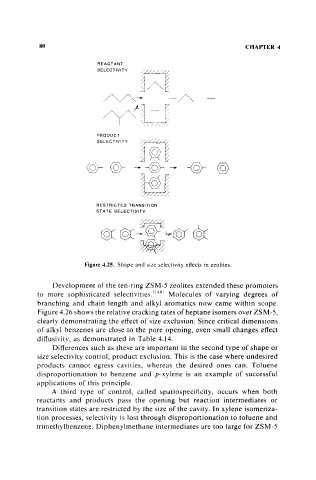
Figure 4.25. Shape and size selectivity effects in zeolites.
Development of the ten-ring ZSM-5 zeolites extended these promoters
to more sophisticated selectivities.' 140) Molecules of varying degrees of
branching and chain length and alkyl aromatics now came within scope.
Figure 4.26 shows the relative cracking rates of heptane isomers over ZSM-5,
clearly demonstrating the effect of size exclusion. Since critical dimensions
of alkyl benzenes are close to the pore opening, even small changes effect
diffusivity, as demonstrated in Table 4.14.
Differences such as these are important in the second type of shape or
size selectivity control, product exclusion. This is the case where undesired
products cannot egress cavities, whereas the desired ones can. Toluene
disproportionation to benzene and p- xylene is an example of successful
applications of this principle.
A third type of control, called spatiospecificity, occurs when both
reactants and products pass the opening but reaction intermediates or
transition states are restricted by the size of the cavity. In xylene isomeriza-
tion processes, selectivity is lost through disproportionation to toluene and
trimethylbenzene. Diphenylmethane intermediates are too large for ZSM-5