Page 299 - Process Equipment and Plant Design Principles and Practices by Subhabrata Ray Gargi Das
P. 299
11.4 Fractionator 301
(vi) Finding the actual number of trays in the column
Vapour and liquid leaving a real tray do not attain equilibrium, i.e., in reality y n and x n will not be
located on the equilibrium curve. This is accounted for by inclusion of Murphree tray efficiency
ðh Þ, in the McCabeeThiele construction procedure.
M
The Murphree tray efficiency ðh Þ, i.e., the efficiency on individual trays is defined for a given
M
component and is equal to the change in composition in the phase divided by the change predicted
by equilibrium.
Mathematically,
Enrichment in vapour composition achieved in the actual tray
M
h ¼
Maximum possible enrichment based on equilibrium being achieved
y n y nþ1
(11.5)
¼
y y nþ1
n
is the Murphree vapour efficiency, y is the vapour mole fraction in
where, on the nth tray, h M n
equilibrium with liquid composition x n and y n is the actual vapour mole fraction leaving tray n. The
component subscript in the equation is dropped because h values are taken to be equal for the two
M
components of a binary mixture.
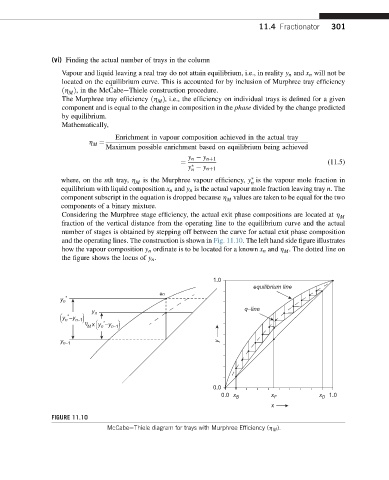
Considering the Murphree stage efficiency, the actual exit phase compositions are located at h M
fraction of the vertical distance from the operating line to the equilibrium curve and the actual
number of stages is obtained by stepping off between the curve for actual exit phase composition
and the operating lines. The construction is shown in Fig. 11.10. The left hand side figure illustrates
how the vapour composition y n ordinate is to be located for a known x n and h . The dotted line on
M
the figure shows the locus of y n .
1.0
equilibrium line
#n
*
y
n
q–line
y
* n
*
y –y n–1
n
M x y –y n–1
n
y n–1 y
0.0
1.0
x x
0.0 x B
F D
x
FIGURE 11.10
McCabeeThiele diagram for trays with Murphree Efficiency (h M ).