Page 186 - Radiochemistry and nuclear chemistry
P. 186
170 Radiochemistry and Nuclear Chemistry
BREMSSTRAHLUNG
8-TRACK OF SECONDARY
BRANCHING TRACK ELECTRON OF > 100 eV
< 8000 eV
TRACK > 6000 eV
COLUMN IONIZATION (~ SPUR < 100 eV
ALONG PRIMARY TRACK
IONIZING
ca. 2nm I _ _ . . . . PROJECTILE
"4 ca. 30 eV HOT ATOM
SPUR FROM SECONDARY ELECTRON
OF 10 - 100 eV (CA. 1-3 ION PAIRS AND
:1-10 EXCITED ATOMS PER 600 nmTRACK
LENGTH). ALSO CALLED A BLOB
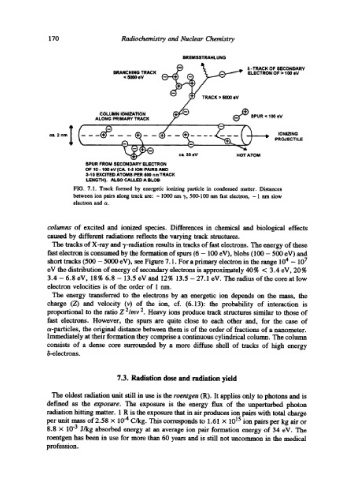
FIG. 7.1. Track formed by energetic ionizing particle in condensed matter. Distances
between ion pairs along track are: -- 1000 nm 3', 500-100 nm fast electron, -- 1 run slow
electron and c~.
columns of excited and ionizeA species. Differences in chemical and biological effects
caused by different radiations reflects the varying track structures.
The tracks of X-ray and ~,-radiation results in tracks of fast electrons. The energy of these
fast electron is consumed by the formation of spurs (6 - 100 eV), blobs (100 - 500 eV) and
short tracks (500 - 5000 eV), see Figure 7.1. For a primary electron in the range 104 - 107
eV the distribution of energy of secondary electrons is approximately 40 % < 3.4 eV, 20 %
3.4 -6.8 eV, 18% 6.8 - 13.5 eV and 12% 13.5 - 27.1 eV. The radius of the core at low
electron velocities is of the order of 1 nm.
The energy transferred to the electrons by an energetic ion depends on the mass, the
charge (Z) and velocity (v) of the ion, of. (6.13): the probability of interaction is
proportional to the ratio Z 2/mv 2. Heavy ions produce track structures similar to those of
fast electrons. However, the spurs are quite close to each other and, for the case of
c~-particles, the original distance between them is of the order of fractions of a nanometer.
Immediately at their formation they comprise a continuous cylindrical column. The column
consists of a dense core surrounded by a more diffuse shell of tracks of high energy
6-electrons.
7.3. Radiation dose and radiation yield
The oldest radiation unit still in use is the roentgen (R). It applies only to photons and is
defined as the exposure. The exposure is the energy flux of the unperturbed photon
radiation hitting matter. 1 R is the exposure that in air produces ion pairs with total charge
per unit mass of 2.58 x 10 -4 C/kg. This corresponds to 1.61 x 1015 ion pairs per kg air or
8.8 • 10 -3 J/kg absorbed energy at an average ion pair formation energy of 34 eV. The
roentgen has been in use for more than 60 years and is still not uncommon in the medical
profession.