Page 191 - Radiochemistry and nuclear chemistry
P. 191
Radiation Effects on Matter 175
resistant. Two examples are UO 2 and UC, whose insensitivity to radiation has led to their
use as reactor fuels. In the fuel dements of commercial water cooled reactors UO 2 is used
in the form of small, sintered pellets about 1 cm 3 in volume. Because of the build-up of
pressure from fission gases, these pellets crack at high fluxes (>_ 1022 n/cm2); Figure 7.4.
Another binary compound, CO 2, which is used as a coolant in some older reactors,
decomposes by irradiation to graphite and polymeric species.
In mixtures of inorganic compounds many unexpected and even undesirable reactions may
occur. For example, radiolysis of liquid air (often used in radiation research) yields ozone,
while radiation of humid air yields HNO 3. One of the first observations of radiation-induced
changes was the darkening of glass. Glass often contains iron, manganese, and other metals
that can exist in several oxidation states with different colors. As a result of the irradiation,
the oxidation state can change, resulting in change in color. Dislocations as well as trapped
electrons also contribute to the color changes in glass. In chemical and metallurgical work
with highly active substances it is desirable to observe the experiment through a thick glass
window, which provides protection from the radiation. In order to avoid the coloring of the
glass, a small amount (1 - 2 %) of an element which can act as an electron trap is added,
e.g. CeO 2, which acts by the reaction Ce 4+ + e- --, Ce 3+. After an exposure of 104 Gy
the transmission to light of ordinary glass had been reduced to 44 %, while for a CeO 2
protected glass it was still 89 %.
Glass is very resistant to radiation damage because it is a noncrystalline solid liquid.
Therefore, in such a material, it is not possible to speak of dislocations: the random
structure of the glass allows it to include foreign species throughout the sample. This is why
glass has been intensely studied, including full scale tests, as the matrix ('container') for
high active waste (HAW) consisting of fission products and actinides.
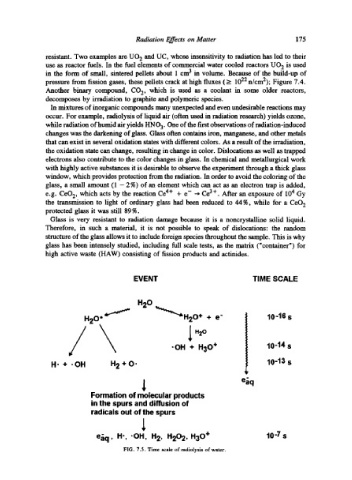
EVENT TIME SCALE
H20
+ e"
// ~"~H20+ 1 "'o 10-16 s
H20
-OH + H30 §
10-14 s
H" + 9 OH H 2 + O' 10-13 s
eiq
Formation of molecular products
in the spurs and diffusion of
radicals out of the spurs
eiq, H-, .OH, H 2, H202, H30 + 10-7 s
FIG. 7.5. Time scale of radiolysis of water.