Page 195 - Radiochemistry and nuclear chemistry
P. 195
Radiation Effects on Matter 179
.' 411' ,Ill m n W u ~mmLstt.
1.6 ~Md
1.4 ! 9 m m Ditmmm
I I' 9 la:Oioll ~ I, qloU, alll Illl
i __ - -
1.2 I : ,
0
E
-~. 1.0
II 9 Ibmilmlw ~ ~ Ir,4s~
E3 ; i ~(-,W
.-I \ ~ ' =
LLI
7- 0.8 "- Irmmlmol
z i 9 SCkd~ mallon' II(..~I
0 -- -' Nq i moils
I--
_< 0.6 e-ms
p
,r . ..
nt
II Ima W l~ll~r X-rays
O.4 ..... ! i i - U Lefoll x*m~
t i , !
GI 0 i~r ,m stttir x.,~ km
0 lira. it Sd,~
Ii ~,, ,.,,~ 1
0.2 .....
..~ . ! ~1. 1 ~ lltmu lit iltmlke Icln~
l MI~ kla LET torture
0.(2 , I ~
0.1 1 10 100
dEabs/dx (eV/nm)
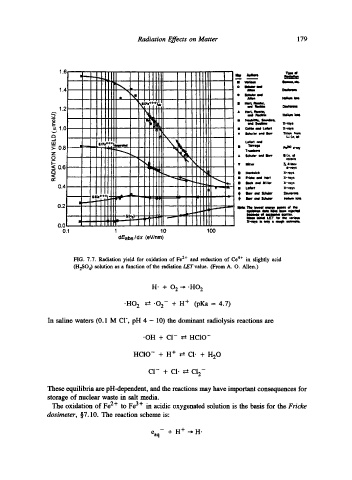
FIG. 7.7. Radiation yield for oxidation of Fe 2+ and reduction of Ce 4+ in slightly acid
(H2SO 4) solution as a function of the radiation LET value. (From A. O. Allen.)
H- + 0 2 --~-HO 2
9 HO 2 ~.O 2 - + H + (pKa = 4.7)
In saline waters (0.1 M CI-, pH 4 - 10) the dominant radiolysis reactions are
9 OH + CI- ~ HCIO-
HCIO- + H + ~-el. + H20
CI- + CI. ~ CI 2-
These equilibria are pH-dependent, and the reactions may have important consequences for
storage of nuclear waste in salt media.
The oxidation of Fe 2 + to Fe 3 + in acidic oxygenated solution is the basis for the Fricke
dosimeter, w The reaction scheme is:
eaq + H + -,H"