Page 198 - Radiochemistry and nuclear chemistry
P. 198
182 Radiochemistry and Nuclear Chemistry
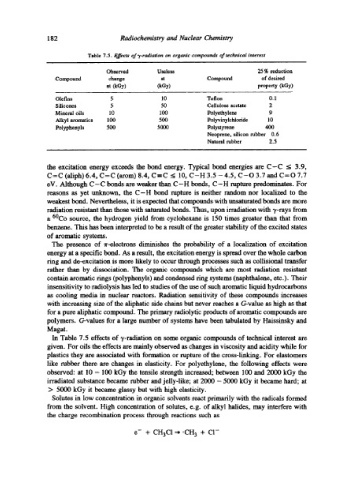
Table 7.5. Effects of-/-radiation on organic compounds of technical interest
Observed Useless 25 % reduction
Compound change at Compound of desired
at (kGy) (kGy) property (kGy)
Olefins 5 10 Teflon 0.1
Silicones 5 50 Cellulose acetate 2
Mineral oils 10 100 Polyethylene 9
Alkyl aromatics 100 500 Polyvinylchloride 10
Polyphenyls 500 5000 Polystyrene 4130
Neoprene, silicon rubber 0.6
Natural rubber 2.5
the excitation energy exce.exls the bond energy. Typical bond energies are C-C < 3.9,
C=C (aliph) 6.4, C=C (arom) 8.4, C--C _< 10, C-H 3.5 - 4.5, C-O 3.7 and C=O 7.7
eV. Although C-C bonds are weaker than C-H bonds, C-H rupture predominates. For
reasons as yet unknown, the C-H bond rupture is neither random nor localized to the
weakest bond. Nevertheless, it is expected that compounds with unsaturated bonds are more
radiation resistant than those with saturated bonds. Thus, upon irradiation with "y-rays from
a 6~ source, the hydrogen yield from cyclohexane is 150 times greater than that from
benzene. This has been interpreted to be a result of the greater stability of the excited states
of aromatic systems.
The presence of r-electrons diminishes the probability of a localization of excitation
energy at a specific bond. As a result, the excitation energy is spread over the whole carbon
ring and de-excitation is more likely to occur through processes such as collisional transfer
rather than by dissociation. The organic compounds which are most radiation resistant
contain aromatic rings (polyphenyls) and condensed ring systems (naphthalene, etc.). Their
insensitivity to radiolysis has led to studies of the use of such aromatic liquid hydrocarbons
as cooling media in nuclear reactors. Radiation sensitivity of these compounds increases
with increasing size of the aliphatic side chains but never reaches a G-value as high as that
for a pure aliphatic compound. The primary radiolytic products of aromatic compounds are
polymers. G-values for a large number of systems have been tabulated by Haissinsky and
Magat.
In Table 7.5 effects of ~,-radiation on some organic compounds of technical interest are
given. For oils the effects are mainly observed as changes in viscosity and acidity while for
plastics they are associated with formation or rupture of the cross-linking. For elastomers
like rubber there are changes in elasticity. For polyethylene, the following effects were
observed: at 10 - 100 kGy the tensile strength increased; between 100 and 2000 kGy the
irradiated substance became rubber and jelly-like; at 2000 - 5000 kGy it became hard; at
> 5000 kGy it became glassy but with high elasticity.
Solutes in low concentration in organic solvents react primarily with the radicals formed
from the solvent. High concentration of solutes, e.g. of alkyl halides, may interfere with
the charge recombination process through reactions such as
e- + CH3CI--, .CH 3 + CI-