Page 194 - Radiochemistry and nuclear chemistry
P. 194
178 Radiochemistry and Nudear Chemistry
0.5 I I I!i!il
.... .jl,o, o.,, , so, I
~ 04 i
0
C
~r
,--, 0.3 ~,,~ ,
,,,-I
t,,kl
,
~ 0.2
t'-"-
0H202
~ O.t
Fi OH2
0 _ ._..,.,a,,.~ -, - ,-.,~-
O.t I tO tO0
Eobs/dx (eV/nn)
0.4
! I IIIIII
u
O
=r 0.3
v
..J
LI.I ",,. ~G H
>- 0.2
,, ~.~ ~. ~ .,.--
I'-
0.1
0H202.. ~. ~- --"
.
0
0.1 I IO IOO
Eob$1dx (eVlnn)
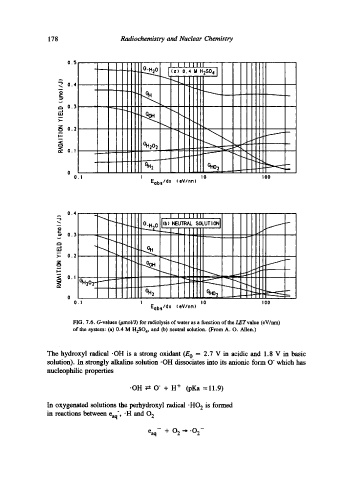
FIG. 7.6. G-values (/unol/J) for radiolysis of water as a function of the LET value (eV/nm)
of the system: (a) 0.4 M H2SO 4, and (b) neutral solution. (From A. O. Allen.)
The hydroxyl radical .OH is a strong oxidant (E 0 = 2.7 V in acidic and 1.8 V in basic
solution). In strongly alkaline solution .OH dissociates into its anionic form O- which has
nucleophilic properties
9 OHm-O- + H + (pKa-11.9)
In oxygenated solutions the perhydroxyl radical -HO 2 is formed
in reactions between eaq, "H and 0 2
m
gaq + 02 "} "02