Page 258 - Radiochemistry and nuclear chemistry
P. 258
242 Radiochemistry and Nuclear Chemistry
In the remainder of this paragraph we discuss the behavior of trace level concentrations
as it is desirable in some applications to use very low concentrations of radiotracers with
no carrier.
9.2.1. Adsorption
Solutes in contact with surfaces have a tendency to be adsorbed on the surface. In order
to cover the glass surface of a one liter vessel with a monomolecular layer of a hydrated
cation only 10 .7 - 10 -8 moles are required. As indicated in the previous paragraph, the
amount of radionuclide in the solution may be less than this and, in principle, all the
radioactive atoms could be adsorbed on the walls of the vessel. The Paneth and Fajans rule
for tracer adsorption states that: "a micro component is adsorbed on a solid macro
component or precipitated together with it if it forms an insoluble compound with a counter
ion of the macro component'.
The amount of radionuclide that is adsorbed on the walls of the container depends on the
concentration, on the chemical state of the radionuclide and on the nature of the container
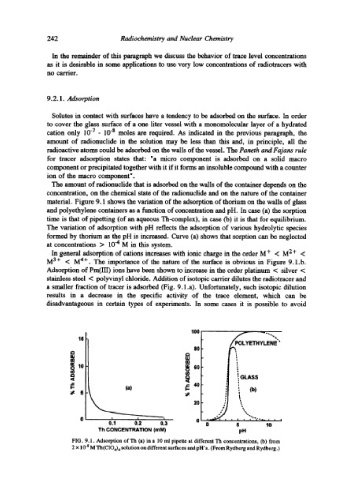
material. Figure 9.1 shows the variation of the adsorption of thorium on the walls of glass
and polyethylene containers as a function of concentration and pH. In case (a) the sorption
time is that of pipetting (of an aqueous Th-complex), in case (b) it is that for equilibrium.
The variation of adsorption with pH reflects the adsorption of various hydrolytic species
formed by thorium as the pH is increased. Curve (a) shows that sorption can be neglected
at concentrations > 10 -4 M in this system.
In general adsorption of cations increases with ionic charge in the order M + < M 2 + <
M 3+ < M 4+. The importance of the nature of the surface is obvious in Figure 9.1.b.
Adsorption of Pm(III) ions have been shown to increase in the order platinum < silver <
stainless steel < polyvinyl chloride. Addition of isotopic carrier dilutes the radiotracer and
a smaller fraction of tracer is adsorbed (Fig. 9.1.a). Unfortunately, such isotopic dilution
results in a decrease in the specific activity of the trace element, which can be
disadvantageous in certain types of experiments. In some cases it is possible to avoid
100
16
8O
o r~
uJ uJ t
m m 0 !
nc 10 ~ 60 0
o
a s !
: GLASS
-- 411 t '; (b)
&=
6
20
""--'--'-"---'--i
I I I I I " "l" " ,ll. I ! I
0.1 0.2 0.3 0 5 10
Th CONCENTRATION (mM) pH
FIG. 9.1. Adsorption of Th (a) in a 10 ml pipette at different Th concentrations, (b) from
2 x 10 .8 M Th(CIO4) 4 solution on different surfaces and pH's. (From Rydberg and Rydberg.)