Page 263 - Radiochemistry and nuclear chemistry
P. 263
Uses of Radioactive Tracers 247
E% = IOO D/(D + 1) (9.5)
where D is the distribution ratio of the radioactivity between the two phases
D = Rorg/Raq = $org/lorg/~aqAaq ~ Norg/Naq = [M]org/[M]aq (9.6)
The last equality requires that the radioactive measurements R are carried out on equal
phase volumes (eventually evaporated to dryness) and that fforg = ~kaq, a requirement easily
met by proper choice of radiometric equipment. Thus the D-value directly reflects the
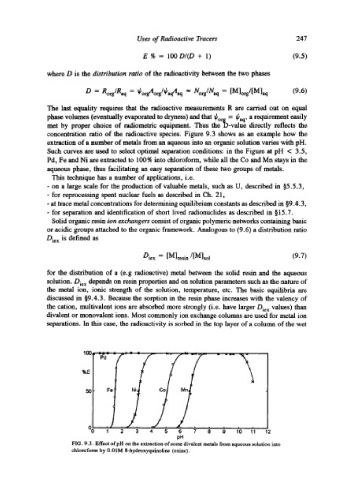
concentration ratio of the radioactive species. Figure 9.3 shows as an example how the
extraction of a number of metals from an aqueous into an organic solution varies with pH.
Such curves are used to select optimal separation conditions: in the Figure at pH < 3.5,
Pd, Fe and Ni are extracted to 100 % into chloroform, while all the Co and Mn stays m the
aqueous phase, thus facilitating an easy separation of these two groups of metals.
This technique has a number of applications, i.e.
- on a large scale for the production of valuable metals, such as U, described in {}5.5.3,
- for reprocessing spent nuclear fuels as described in Ch. 21,
- at trace metal concentrations for determining equilibrium constants as described in w
- for separation and identification of short lived radionuclides as described in w 15.7.
Solid organic resin ion exchangers consist of organic polymeric networks containing basic
or acidic groups attached to the organic framework. Analogous to (9.6) a distribution ratio
Die x is defined as
Die x = [M]resi n/[M]sol (9.7)
for the distribution of a (e.g radioactive) metal between the solid resin and the aqueous
solution. Die x depends on resin properties and on solution parameters such as the nature of
the metal ion, ionic strength of the solution, temperature, etc. The basic equilibria are
discussed in w Because the sorption in the resin phase increases with the valency of
the cation, multivalent ions are absorbed more strongly (i.e. have larger Die x values) than
divalent or monovalent ions. Most commonly ion exchange columns are used for metal ion
separations. In this case, the radioactivity is sorbM in the top layer of a column of the wet
Pd . . . . .
%E
5O
2 3 5 6 7 8 9 10 11 12
pH
FIG. 9.3. Effect ofpH on the extraction of some divalent metals from aqueous solution into
chloroform by 0.01M 8-hydroxyquinoline (oxine).