Page 265 - Radiochemistry and nuclear chemistry
P. 265
Uses of Radioactive Tracers 249
/
S " /' Z'
4 ........ ~-..-v--..v .... ~.--v .... v-'---r /
m
o" |
. / i _ /
),- ! ,e
I"
m
I---
r
<.~
I.I.
<.)
ILl
Q. 9 o ~ /
-"
uO
-- v "i vi
!
l !
m !
b' o'
MILLILITER OF TITRANT
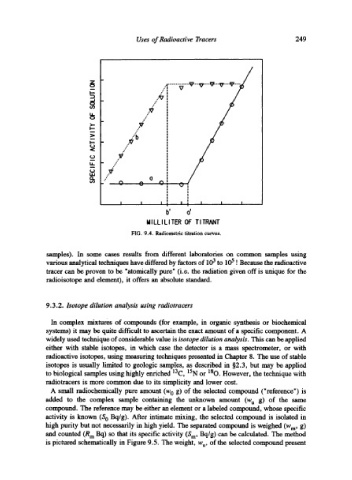
FIG. 9.4. Radiometric titration curves.
samples). In some cases results from different laboratories on common samples using
various analytical techniques have differed by factors of 103 to 105 ! Because the radioactive
tracer can be proven to be "atomically pure" (i.e. the radiation given off is unique for the
radioisotope and element), it offers an absolute standard.
9.3.2. Isotope dilution analysis using radiotracers
In complex mixtures of compounds (for example, in organic synthesis or biochemical
systems) it may be quite difficult to ascertain the exact amount of a specific component. A
widely used technique of considerable value is isotope dilution analysis. This can be applied
either with stable isotopes, in which case the detector is a mass spectrometer, or with
radioactive isotopes, using measuring techniques presented in Chapter 8. The use of stable
isotopes is usually limited to geologic samples, as describe~ in w but may be applied
to biological samples using highly enriched 13C, 15N or 180. However, the technique with
radiotracers is more common due to its simplicity and lower cost.
A small radiochemically pure amount (w 0 g) of the selected compound ('reference') is
added to the complex sample containing the unknown amount (w u g) of the same
compound. The reference may be either an element or a labeled compound, whose specific
activity is known (S O Bq/g). After intimate mixing, the selected compound is isolated in
high purity but not necessarily in high yield. The separated compound is weighed (w m, g)
and counted (R m Bq) so that its specific activity (S m, Bq/g) can be calculated. The method
is pictured schematically in Figure 9.5. The weight, w u, of the selected compound present