Page 270 - Radiochemistry and nuclear chemistry
P. 270
254 Radiochemistry and Nuclear Chemistry
painting entitled "Christ and Magdalene" done in the Old Dutch style was proved to be a
twentieth century forgery when NAA showed < 7 ppm silver and < 1.3 ppm antimony
in the white lead paint. The sixteenth and seventeenth century Dutch paintings have white
lead with about 10 - 1000 ppm silver and 50 - 230 ppm antimony.
It has been found that hair contains trace metals (e.g., Cu, Au, Ce, Na) in ratios which
are typical for a particular individual, and activation analysis can be used to identify hair
from a particular person. This application achieved public notice when it was found that
hair from Napoleon had a relatively large amount of arsenic, indicating that some time prior
to his death he had received large doses of arsenic. Through analysis of the hair of the
Swedish king Erik XIV (who died suddenly in 1577 after a meal of pea-soup) it has been
found that he must have received lethal amounts of arsenic as well as large amounts of
mercury. The latter is assumed to have been taken into his body through the use of a
mercury compound for treatment of an old wound.
The high sensitivity of activation analysis has made it very useful in environmental
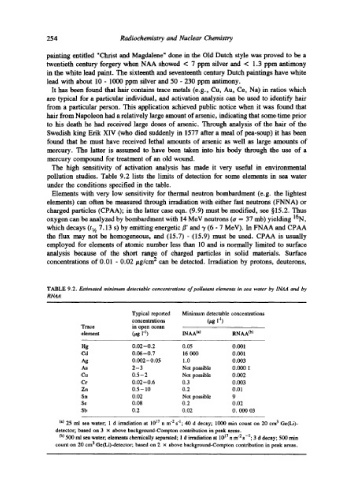
pollution studies. Table 9.2 lists the limits of detection for some elements in sea water
under the conditions specified in the table.
Elements with very low sensitivity for thermal neutron bombardment (e.g. the lightest
elements) can often be measured through irradiation with either fast neutrons (FNNA) or
charged particles (CPAA); in the latter case eqn. (9.9) must be modified, see w Thus
oxygen can be analyzeA by bombardment with 14 MeV neutrons (o = 37 mb) yielding 16N,
which decays (tl/~ 7.13 s) by emitting energetic 3- and "y (6 - 7 MeV). In FNAA and CPAA
the flux may not be homogeneous, and (15.7) - (15.9) must be used. CPAA is usually
employed for elements of atomic number less than 10 and is normally limited to surface
analysis because of the short range of charged particles in solid materials. Surface
concentrations of 0.01 - 0.02/~g/cm 2 can be detected. Irradiation by protons, deuterons,
TABLE 9.2. Estimated minimum detectable concentrations of pollutant elements in sea water by IN,4_A and by
RNAA
Typical reported Minimum detectable concentrations
concentrations ~g i q)
Trace in open ocean
element (jag 1 "l) INAA (a) RNAA (b)
Hg 0.02-0.2 0.05 0.001
Cd 0.06-0.7 16 000 0.001
Ag 0.002-0.05 1.0 0.003
As 2-3 Not possible 0.000 1
Cu 0.5 - 2 Not possible 0.002
Cr 0.02-0.6 0.3 0.003
Zn 0.5-10 0.2 0.01
Sn 0.02 Not possible 9
Se 0.08 0.2 0.02
Sb 0.2 0.02 O. 000 03
(*) 25 ml sea water; 1 d irradiation at 1017 n m 2 s "1" 40 d decay; 1000 rain count on 20 cm 3 Ge(Li)-
detector; based on 3 x above background-Compton contribution in peak areas.
(b) 500 ml sea water; elements chemically separated; 1 d irradiation at 1017 n m "2 s -l" 3 d decay; 500 min
count on 20 cm 3 GeCLi)-detector; based on 2 x above background-Compton contribution in peak areas.