Page 43 - Radiochemistry and nuclear chemistry
P. 43
32 Radiochemistry and Nuclear Chemistry
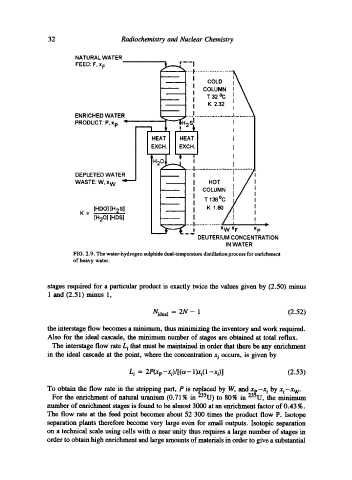
NATURAL WATER
FEED: F, x F
: co.0
COLUMN t \
i T32~ I k
K 2.32 I I ~
ENRICHED WATER _..........~ ..) ....................... ~ ............
PRODUCT: P, Xp "
..... J....,. o.....,..4,, o .,, .~..,.. o 9 o.. s .,, e...
DEPLETED WATER I
WASTE: W, x W "= I HOT
I COLUMN
II T 138~
[HDO] [H2S] I K 1.80/
[H20] [HDS] I
! x w x F Xp
..... ~~176176176176176176
.,~176
DEUTERIUM CONCENTRATION
IN WATER
FIG. 2.9. The water-hydrogen sulphide dual-temperature distillation process for enrichment
of heavy water.
stages required for a particular product is exactly twice the values given by (2.50) minus
1 and (2.51) minus 1,
Nidea I -" 2N- 1 (2.52)
the interstage flow becomes a minimum, thus minimizing the inventory and work required.
Also for the ideal cascade, the minimum number of stages are obtained at total reflux.
The interstage flow rate L i that must be maintained in order that there be any enrichment
in the ideal cascade at the point, where the concentration x i occurs, is given by
L i = 2P(xp-xi)/[(a- 1)xi(l -xi) ] (2.53)
To obtain the flow rate in the stripping part, P is replaced by W, and x[,-x i by xi-x w.
hm
For the enric ~ent of natural uranium (0.71% in 235 U) to 80% in 23~U, the minimum
number of enrichment stages is found to be almost 3000 at an enrichment factor of 0.43 %.
The flow rate at the feed point becomes about 52 300 times the product flow P. Isotope
separation plants therefore become very large even for small outputs. Isotopic separation
on a technical scale using cells with a near unity thus requires a large number of stages in
order to obtain high enrichment and large amounts of materials in order to give a substantial