Page 41 - Radiochemistry and nuclear chemistry
P. 41
30 Radiochemistry and Nuclear Chemistry
2.8. Isotope separation processes
Many fields of fundamental science have found great advantage in using pure or enriched
isotopes. The industrial use of nuclear power also requires the enrichment of particular
isotopes, primarily of the uranium fuel. The methods which have been developed to achieve
isotopic fractionation may be divided into two groups.
(a) Equilibrium processes (w These processes consume little energy, but the size of
the isotope effect in normal chemical equilibrium limits their use to the isotope fractionation
of very light elements, usually atomic number less than about 10.
(b) Rate processes (w This includes processes which depend on such phenomena as
ionic mobility, diffusion, electrolysis, electromagnetic separations, centrifugation, and
kinetic processes of a chemical nature. While the isotopic effects in these processes are
normally larger than for equilibrium processes, they require a large amount of energy and
therefore have economic restrictions.
f P,x~ = Xp
LN-1 ]
" XN. 1 N
r- L~+1~Xn+1 L x h
ID
E
t-
o
. i
L~
e-
LU Ln-lgX~ - 1 ,,
F, x F
--Ln-1 -1 Ln'2 Xn'2
L n -__] xh-3~ L~ .2~~. 2x"
e-
.m
L_
Li xi
-------'--="- W, x i = Xw
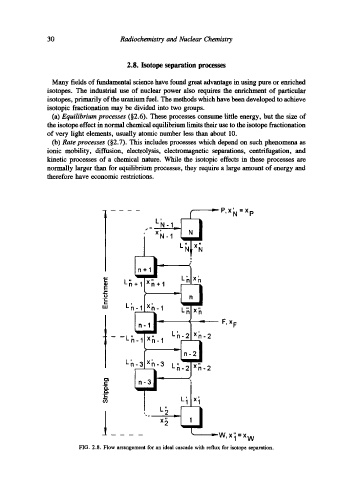
FIG. 2.8. Flow arrangement for an ideal cascade with reflux for isotope separation.